Translate this page into:
Cogan syndrome: An autoimmune eye and ear disease with systemic manifestations
2 Department of Rheumatology and Clinical Immunology, Sir Ganga Ram Hospital, New Delhi, India
Corresponding Author:
Gurbir Singh Bhandari
Department of Rheumatology, Adesh Institute of Medical Sciences and Research, Bathinda, Punjab
India
bhandari.gurbir.singh@gmail.com
| How to cite this article: Bhandari GS, Duggal L, Jain N, Patel J. Cogan syndrome: An autoimmune eye and ear disease with systemic manifestations. Natl Med J India 2019;32:349-351 |
Abstract
Cogan syndrome (CS) is a rare vasculitis seen primarily in young adults. It predominantly affects eyes, ears and the heart with characteristic findings of interstitial keratitis, sensorineural hearing loss and vestibular dysfunction. A high index of suspicion is required to diagnose this rare disorder. It is one of the few vasculitis which can involve vessels of all sizes: small, medium and large. Coexistence of inflammatory bowel disease (IBD) in Cogan syndrome has been described in the literature. Immunosuppressive agents such as corticosteroids with or without steroid sparing agents are the standard of care. Early diagnosis and treatment are the cornerstone of treatment to prevent permanent damage to the ears and eyes. We describe a patient with Cogan syndrome with large vessel vasculitis and IBD. Our patient was treated with glucocorticoids and methotrexate.Introduction
Cogan syndrome (CS) is a variable vessel vasculitis with prominent autoimmune manifestations of the eye, ear and cardiac valves. It is among the few vasculitides which can involve large-, medium- and small-sized vessels. Interstitial keratitis, sensorineural hearing loss and vestibular dysfunction are classical manifestations of CS.[1] It is a rare disease with around 250 cases reported with considerable publication overlap.[2] Although two case reports of children have been reported earlier,[3],[4] there are no reports of adult CS from India. We describe a rare case of CS with not only eye or ear manifestations but also the presence of large vessel vasculitis and inflammatory bowel disease (IBD).
The Case
A 21-year-old male presented with complaints of redness of eyes for 30 days. It was associated with pain, photophobia and headache which increased in the evening. He also complained of tinnitus with involvement of both ears sequentially, more at night and associated with hearing loss. There was a sense of imbalance while running. He had moderate grade fever along with other constitutional symptoms. The patient was admitted to another hospital and was treated with a broad-spectrum antibiotic, injectable steroids, topical steroid eye drops and other supportive medicines.
There was improvement in fever, cough, red eyes, ocular pain and tinnitus, hearing loss (completely recovered) and constitutional symptoms after treatment. He was asymptomatic for few months and again had two similar episodes in the following year. He was again treated with steroids and antibiotics. After three episodes, a hearing aid was prescribed as he had marked loss of hearing.
He was admitted again to another hospital for the fourth attack and was treated with a broad-spectrum antibiotic. Cortico-steroids were not given as he had leucocytosis (total leucocyte count of up to 35 000/cmm). He did not improve, and when he presented to us, he had significant hearing loss, persistent red eyes, fever and few episodes of crampy abdominal pain.
He had already taken antitubercular treatment (ATT) for 9 months for mediastinal lymphadenopathy. On examination of the ear, nose and throat, both air conduction and bone conduction were absent. On eye examination, he had prominent scleral vessels and few black spots on the sclera in both eyes [Figure - 1]. Fundoscopy was normal and slit-lamp examination was suggestive of sclerokeratitis. Previous audiograms were suggestive of significant bilateral sensorineural hearing loss [Figure - 2]. Previous butterfly chart for vestibular function was not suggestive of any vestibular dysfunction, and complete blood count showed leucocytosis and thrombocytosis. His acute phase reactants were raised. Antinuclear antibodies, Rheumatoid factor and antineutrophil cytoplasm antibodies were negative. Complement and anticholinesterase levels were normal. Hepatitis B and C virus, HIV, venereal disease research laboratory test and screen for tuberculosis (Mantoux test, interferon-gamma release assay and computed tomography chest) were negative. Cerebrospinal fluid studies were normal. Ultrasonography of the abdomen showed hepatosplenomegaly with mild wall thickening in the caecum and ascending colon. Magnetic resonance imaging of the brain and orbit were normal. Colonoscopy was done which showed longitudinal ulcers in the caecum and ascending colon along with oedematous mucosa giving pesudopolypoid appearance [Figure - 3]. Biopsy was taken and it showed non-specific ileocolitis. Positron emission tomography CT showed circumferential mural thickening involving supra-renal/infra-renal abdominal aorta and bilateral common iliac artery with aneurysmal dilatation (medium to large vessels) at places suggestive of an inflammatory pathology. There was fluorodeoxyglucose (FDG) avid mural thickening involving the ileocaecal junction and adjacent ascending colon with few mildly FDG avid mesenteric lymph nodes likely infective/ inflammatory [Figure - 4].
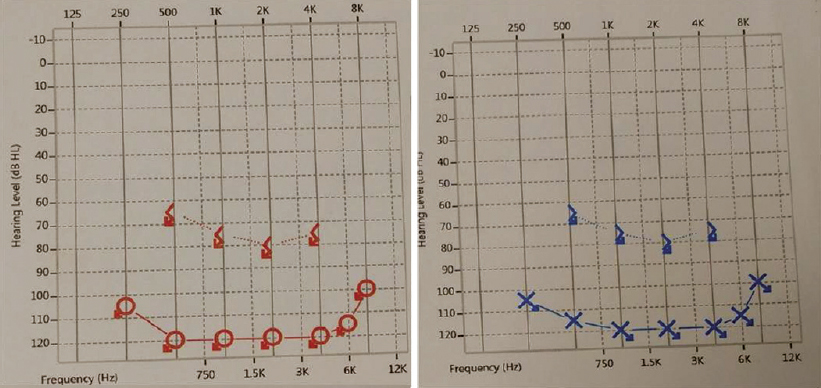 |
| Figure 1: Audiogram depicting hearing loss |
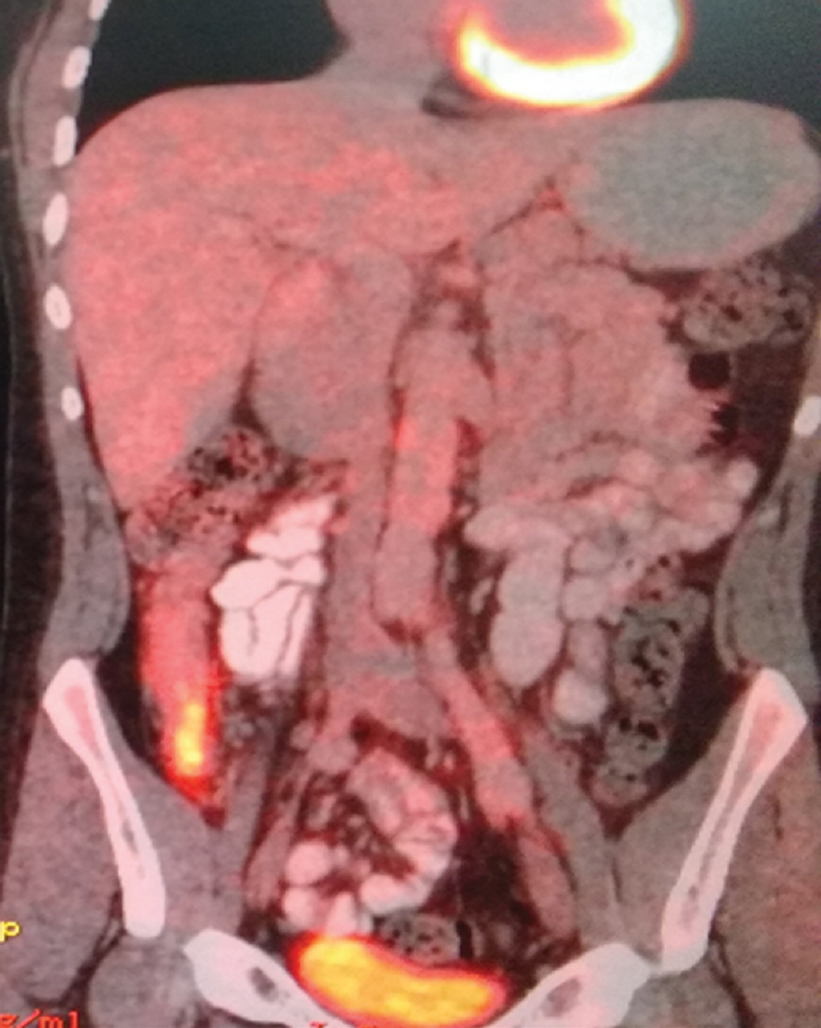 |
| Figure 2: Positron emission tomography-computed tomography suggestive of increased fluorodeoxyglucose uptake in ileocaecal region, abdominal aorta and its major branches along with irregularities in the above-mentioned vessels |
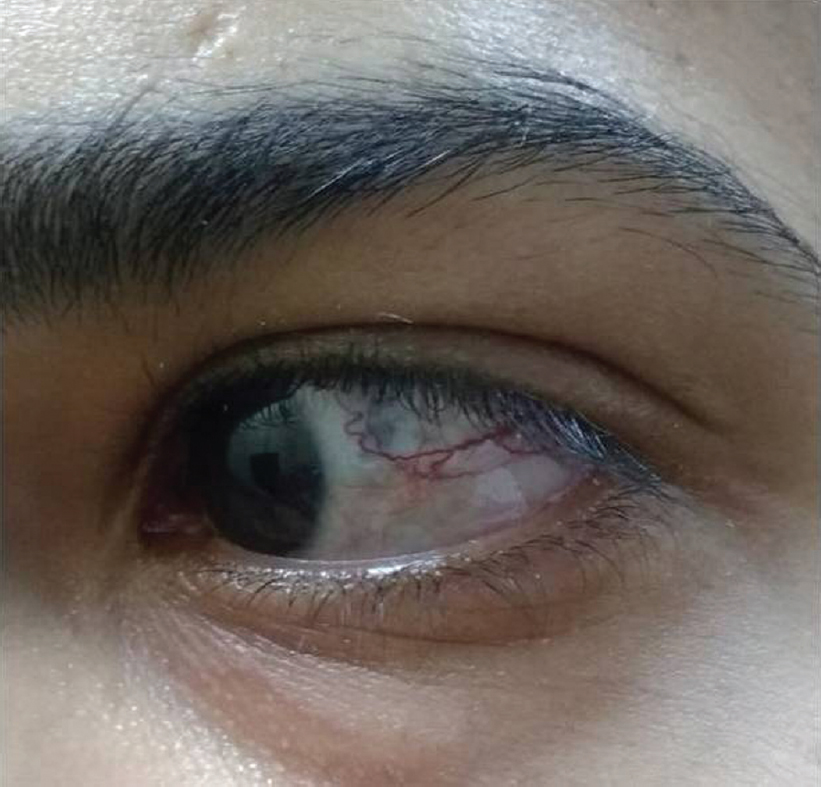 |
| Figure 3: Prominent scleral vessels and black spots |
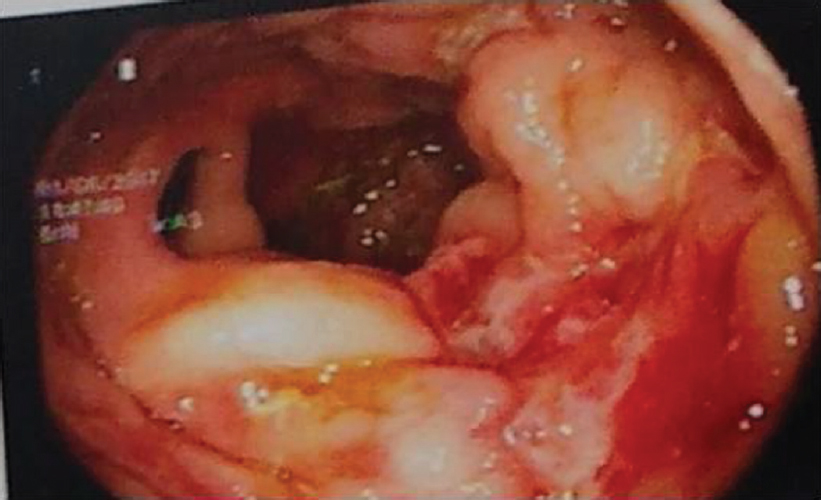 |
| Figure 4: Colonoscopy showing longitudinal ulcers in caecum and ascending colon |
Hence, this was a case of Cogan syndrome (CS) with sclerokeratitis, sensorineural hearing loss, Takayasu-like large vessel vasculitis and IBD. The patient was given high-dose corticosteroids: first by the intravenous route followed by oral steroids. Injection methotrexate (20 mg s.c.) was started because of the large vessel vasculitis and systemic manifestations of the disease. His symptoms improved except the hearing loss, and he is currently under treatment (low-dose corticosteroid and methotrexate).
Discussion
Cogan syndrome is a rare disorder characterized by recurrent inflammation of the cornea and inner ear structures. It was first described by an ophthalmologist Cogan in 1945.[5] The aetiology is not known although autoimmune cause may be considered as antibodies to inner ear structures were found in patients' sera. CS usually affects young adults in their 2nd or 3rd decade. Cigarette smoking is known to be an additional trigger.[6]
The presenting symptoms may be of the vestibuloauditory system in 46% of patients, the ocular system in 38% and both in 15%. However, by the end of 5 months, almost 75% of patients have both eye and ear involvement irrespective of initial presentation. Bilateral interstitial keratitis is a predominant finding; although corneal stromal keratitis, episcleritis, scleritis, uveitis, papillitis, vitritis and choroiditis can occur. The patient may have red eyes, foreign body sensation, pain, photophobia, double vision or decreased vision. However, vision loss occurs in <5% of patients.
Vestibuloauditory symptoms include sensorineural hearing loss, tinnitus and vertigo. A vague unsteadiness of gait can persist in some patients and evidence of vestibular damage can be found in all patients. Mild-to-moderate hearing loss occurs in almost all patients with CS (cochlear hydrops), but profound loss occurs in two-thirds of patients (up to 52%).[7]
Patients may present with claudication if it involves the aorta or its branches. CS may have large- and/or medium-sized vessel vasculitis in 10%-15% of patients (resembles Takayasu's arteritis). Fluttering of anterior mitral leaflet, thickening of valve cusps, left ventricular enlargement, arrhythmias, pericarditis and myocardial infarction can also occur rarely. Sometimes, CS may present as medium vessel predominant vasculitis as in polyarteritis nodosa.[8] CS may involve the central nervous system rarely, and sometimes, it is associated with interstitial nephritis, hypothyroidism, sarcoidosis, ulcerative colitis or Crohn disease.
The largest case series of 22 concomitant CS-IBD patients showed that in more than 50% of CS patients IBD was asymptomatic. It also showed that immunomodulatory treatment for IBD (even antitumour necrosis factor [TNF] therapy) does not prevent onset of CS. However, IBD activity does not correlate with CS activity.[9] Our patient had rare manifestations in the form of IBD and large vessel involvement apart from the eye and ear manifestations.
The diagnosis of CS is not difficult as classical non-syphilitic interstitial keratitis or acute to subacute onset hearing loss in a young patient raises suspicion of CS. Syphilis, Lyme disease, Epstein–Barr virus infection, chlamydia and Whipple disease are the differential diagnosis among the infectious group. Behcet disease, relapsing polychondritis, sarcoidosis and Sjögren syndrome are a few autoimmune disorders that should be excluded.
As CS is a rare disease, there are no standard guidelines for management. Patients should be treated with an adequate dose of corticosteroids along with team management. Initial management should be with systemic corticosteroids in a dose of 1–2 mg/kg/day. Steroid-sparing drugs are often used such as methotrexate, leflunomide, azathioprine, tacrolimus, cyclophosphamide and anti-TNF.[2] In CS with large or medium vessel vasculitis, cyclophosphamide and cyclosporine are used; although we used methotrexate which also has an effect in large vessel vasculitis such as Takayasu arteritis.
Conclusion
Ear involvement in CS is important as early treatment with high-dose corticosteroid can effectively prevent permanent hearing loss. A thorough cardiovascular examination should be done. IBD (silent or overt) can be associated with CS. Thus, it should be ruled out with colonoscopy and managed accordingly. In patients with more than two episodes of hearing loss, immunosuppressants should be started as early as possible to prevent further relapse and consequent ear damage.
Conflicts of interest. None declared
| 1. | Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised International Chapel Hill consensus conference nomenclature of vasculitides. Arthritis Rheum 2013;65:1–11. [Google Scholar] |
| 2. | Mazlumzadeh M, Matteson EL. Cogan's syndrome: An audiovestibular, ocular, and systemic autoimmune disease. Rheum Dis Clin North Am 2007;33:855–74, vii–viii. [Google Scholar] |
| 3. | Juneja M, Jain R, Chakarbarty B. Atypical Cogan syndrome mimicking acute rheumatic fever. Indian Pediatr 2011;48:561–3. [Google Scholar] |
| 4. | Kothari VM, Shah N, Venkatachalam S, Balakrishnan C, Mangat G, Joshi VR. Cogan's syndrome in a nine year old: Case report and review of literature. J Indian Rheum Assoc 1995;3:164–5. [Google Scholar] |
| 5. | Cogan D. Syndrome of nonsyphilitis interstitial keratitis and vestibuloauditory symptoms. Arch Ophthalmol 1945;33:144–9. [Google Scholar] |
| 6. | Gluth MB, Baratz KH, Matteson EL, Driscoll CL. Cogan syndrome: A retrospective review of 60 patients throughout a half century. Mayo Clin Proc 2006;81:483–8. [Google Scholar] |
| 7. | Haynes BF, Kaiser-Kupfer MI, Mason P, Fauci AS. Cogan syndrome: Studies in thirteen patients, long-term follow-up, and a review of the literature. Medicine (Baltimore) 1980;59:426–41. [Google Scholar] |
| 8. | Livingston JZ, Casale AS, Hutchins GM, Shapiro EP. Coronary involvement in Cogan's syndrome. Am Heart J 1992;123:528–30. [Google Scholar] |
| 9. | Vavricka SR, Greuter T, Scharl M, Mantzaris G, Shitrit AB, Filip R, et al. Cogan's syndrome in patients with inflammatory bowel disease: A case series. J Crohns Colitis 2015;9:886–90. [Google Scholar] |
Fulltext Views
3,250
PDF downloads
1,564




