Translate this page into:
Convalescent plasma therapy for Covid-19: A systematic review
2 Department of Neurology, All India Institute of Medical Sciences, Ansari Nagar, New Delhi 110029, India
3 Department of Medical Oncology, All India Institute of Medical Sciences, Ansari Nagar, New Delhi 110029, India
4 Department of Gastroenterology and Human Nutrition Unit, All India Institute of Medical Sciences, Ansari Nagar, New Delhi 110029, India
5 Department of Pulmonary Medicine, Critical Care and Sleep Disorders, All India Institute of Medical Sciences, Ansari Nagar, New Delhi 110029, India
6 Department of Medicine, All India Institute of Medical Sciences, Ansari Nagar, New Delhi 110029, India
Corresponding Author:
Atul Sharma
Department of Medical Oncology, All India Institute of Medical Sciences, Ansari Nagar, New Delhi 110029
India
atul1@hotmail.com
| How to cite this article: Seth T, Elavarasi A, Sahoo RK, S, Madan K, Nischal N, Soneja M, Garg P, Prasad K, Sharma A. Convalescent plasma therapy for Covid-19: A systematic review. Natl Med J India 2020;33:213-221 |
Abstract
Background. Covid-19 has emerged as a pandemic affecting more than 20 million people till date with few, if any, proven therapy. Convalescent plasma (CP) containing antibodies against the virus has been used with some success. We did a systematic review to synthesize the available data on CP therapy for treatment of Covid-19 to study the efficacy and safety outcomes.Methods. Two reviewers searched the published and pre-published literature between 1 January 2019 and 23 June 2020 for studies comparing the use of CP with standard therapy for Covid-19 patients. Data from the selected studies were abstracted and analysed for efficacy and safety outcomes. Critical appraisal of the evidence was done by using the Joanna Briggs Institute tool and the quality of evidence was graded as per GRADE.
Results. We found 13 case series and 1 randomized trial that fulfilled our search criteria. Of the 12 case series with a total of 264 patients that reported the efficacy outcomes, 11 studies showed favourable results with survival benefit. The only RCT with 103 patients did not show any mortality benefit but was terminated early prior to complete enrolment. A single large study of 5000 patients reported safety outcomes and showed no major adverse events in patient streated with CP.
Conclusion. There is very low-quality evidence to suggest efficacy and safety of CP in patients with Covid-19 infection. Well-designed randomized trials are urgently needed to provide robust data.
Introduction
The history of passive immunotherapy using plasma from patients convalescing from an infection started in 1890s when it was used for the treatment of infectious diseases.[1] The biological rationale being that convalescent plasma (CP) contains antibodies against the specific pathogen which may neutralize the pathogenic organism and modify the inflammatory response of the host to the organism.[2],[3] The effect of CP varies with the severity of disease, dose of antibodies and usually works better in the initial phases of illness or as prophylaxis.[1],[2]
The efficacy of CP was supported by anecdotal evidence from H5NI,[4] H7N9,[5] MERS,[6] and SARS or SARS CoV-1[7] viral infections. The experience from outbreaks of SARS-CoV-1, another corona virus disease, had shown that neutralizing antibodies in CP could reduce viraemia in sick patients.[7],[8],[9],[10] Therefore, in the absence of a pharmacological therapy or a vaccine for Covid-19/SARS-CoV-2, passive antibody administration via CP seems a valid method of reducing viraemia and providing immediate enhanced antibody-mediated immunity to infected patients. However, the risks of passive antibody administration via CP are well known. These include the risk of adverse reactions, transfusion-related acute lung injury (TRALI) and transmission of infections. A theoretical risk of antibody-dependent enhancement of infection (ADE) is also a possibility[10] but seems unlikely as plasma containing high titres of neutralizing antibody against the SARS-CoV-2 are being used.[11]
We did a systematic review of the available studies to determine if the use of CP is effective and safe in reducing mortality in patients with Covid-19 compared to those not given CP.
Methods
Criteria for selecting studies
We sought to identify all clinical studies including randomized controlled trials (RCTs), cohort studies with control groups, observational studies—both prospective and retrospective, and case series for the purpose of this review. We included both published studies as well as pre-print and non-peer reviewed literature as Covid-19 is a new disease and information available on pre-print servers may be valuable in guiding clinical practice given the rapidly increasing number of cases worldwide and a lack of effective therapy. We included papers available in English only. We excluded single case reports from the review.
Types of participants
We included human studies in which patients with confirmed Covid-19 of all ages, sexes and grades of severity were recruited.
Types of intervention
We included studies in which patients with Covid-19 were administered CP collected from patients who had recovered from the infection.
Outcomes
For each study, we sought the following efficacy and safety outcomes:
- Clinical outcomes: Death, improvement in oxygen requirement, and need for mechanical ventilation
- Radiological outcomes: Improvement in computed tomography (CT) chest findings
- Biochemical outcomes: Reduction in C-reactive protein (CRP) and change in interleukin (IL)-6 levels
- Safety outcomes: Adverse effects and complications associated with CP therapy
Search strategy
Two authors (TS and AS) independently searched the PubMed, Embase, Google Scholar and MedRxiv databases using the following search terms: ‘[(Convalescent plasma OR Plasma OR serum) AND (COVID-19 OR SARS-CoV-2)]’ from 1 January 2019 till 23 June 2020. No limits were applied to the search results except studies in humans. Hand searching of cross-references of original articles, reviews and pre-published articles was also done.
Data extraction
The citations were retrieved into a reference management software (Zotero version 5.0.85). Duplicate citations were removed. All the remaining studies were reviewed by going through their title and abstract to select the studies meeting our inclusion criteria mentioned above. Data on outcomes were extracted by one reviewer (TS) and cross-checked by another reviewer (AS). We looked at various aspects of each study such as the type of study, inclusion criteria, dose and timing of CP therapy, additional treatments received, severity and phase of illness, antibody titre and outcomes as mentioned above. The severity of illness was defined as mild, moderate, severe and critical as per the sixth interim edition of diagnosis and treatment protocol of CDC, China.[12]
Assessment of quality of studies
We critically appraised the selected studies with complete information as per the Joanna Briggs Institute (JBI) tool[13] for this systematic review. The evidence was graded using GRADE (Grading of Recommendations, Assessment, Development and Evaluations) methodology.[14]
Results
Description of studies
The search yielded 584 articles on PubMed, 2800 articles on Google scholar, 470 on Embase and 827 on MedRxiv. No additional articles were retrieved on hand searching of references of reviews and original articles. We excluded papers that were commentaries or expert opinions and not clinical studies as they did not fit our inclusion criteria. Single case reports were also excluded. After removal of duplicate citations, we had 14 articles for complete review. We found 13 descriptive studies and only one RCT of CP use for Covid-19 [Table - 1].[15] The PRISMA flow chart is provided as [Figure - 1].

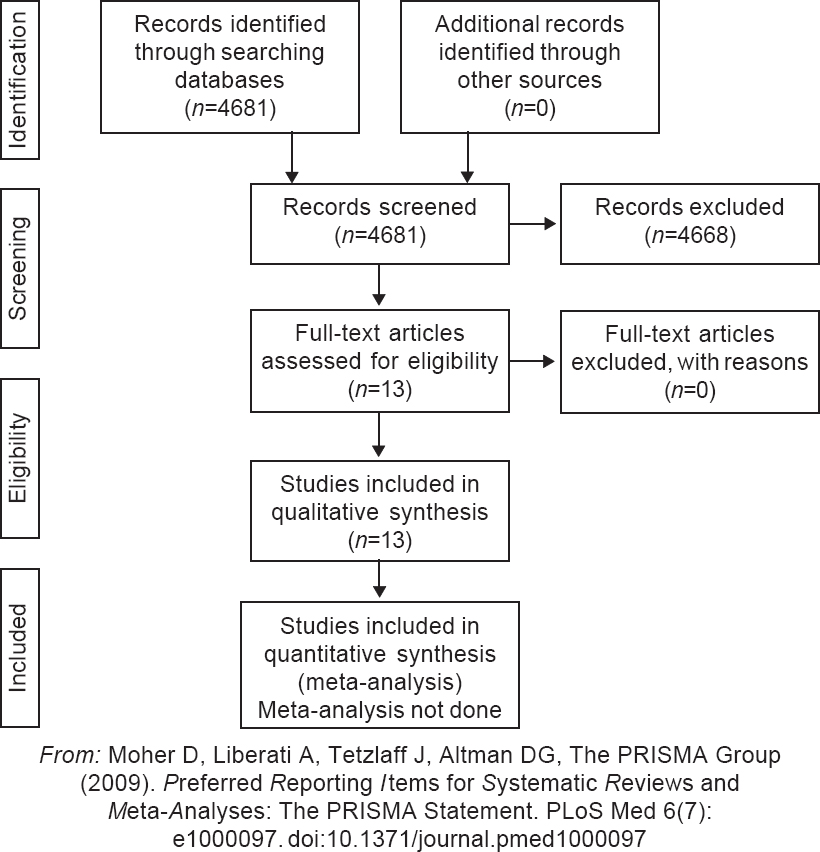 |
| Figure 1: PRISMA flow diagram for study selection |
Risk of bias
An appraisal of the included case series using the JBI tool has been summarized in [Table - 2]. Since the cohort studies had non-blinded assessment of outcomes, they were subject to multiple sources of bias. The risk of bias was low in the RCT.
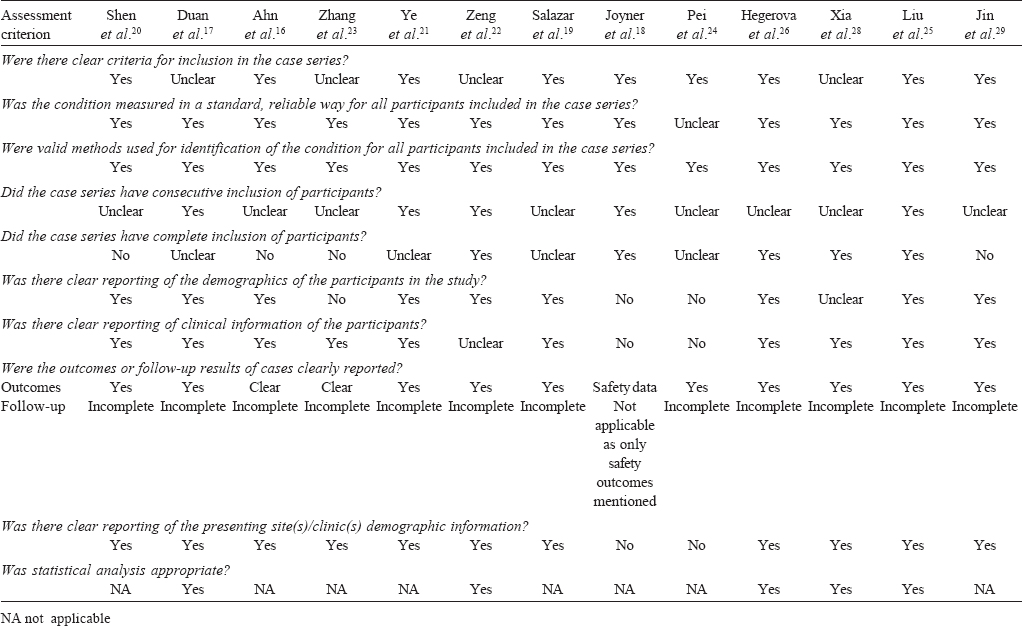
Summary results
A total of 5264 patients included in the 13 cohort studies/ observational studies[16],[17],[18],[19],[20],[21],[22],[23],[24],[25],[26],[27],[28],[29] were treated with CP. Their age range was 18–97 years; the largest study included 5000 patients with severe or life-threatening illness and had 72% patients on mechanical ventilation and 18% in shock. This study reported only safety outcomes. The interval between onset of symptoms and treatment with CP varied from 3 to 38 days; most patients received the therapeutic intervention in the third week of illness [Table - 1]. The dose of CP varied from 200 to 400 ml given 1–3 times over 1–3 days with most patients receiving 2 doses. In one study, the dose was very high, i.e. 2400 ml.[23] The IgG antibody concentration in the CP varied from 1:640 to 1:1000 as reported in two studies.[17],[20] Pre-infusion antibody titres in the treated patients were reported in one study and were 72–217 mg/dl for IgG and 16–273 mg/dl for IgM antibodies.[21] Among the co- medications, all patients received various medications including antivirals, methylprednisolone and intravenous immunoglobulin but had not shown any improvement. The outcomes of each of the series are summarized in [Table - 1].
The RCT included 103 patients (median age 70 years; 60 [58.3%] male) with severe or critical Covid-19. Patients were randomized to CP or standard treatment stratified by disease severity. The recipient patients with high titre of S protein–RBD (receptor binding domain)-specific IgG antibody (≥1:640) were excluded. The donor plasma units with an S-RBD-specific IgG titre of at least 1:640 were used for therapy. The median dose received was 200 ml (IQR 200–300 ml), and 96% of patients received a single dose of plasma infusion. The median interval between the onset of symptoms and randomization was 30 days. Primary outcome was time to clinical improvement within 28 days defined as patient discharged alive or reduction of 2 points on a 6-point disease severity scale. The trial was terminated early after 103 of a planned 200 patients were enrolled because the number of cases fell sharply midway through the trial in China.
Safety outcomes
The largest study of 5000 patients by Joyner et al.[18] focused only on safety profile and adverse events of CP and did not report on efficacy outcomes. Serious adverse events occurred in 36 of 5000 patients in this series. Of these, 15 patients (0.3%) died in less than 4 hours after receiving plasma therapy. Serious adverse events not leading to death included TRALI in 11/5000 patients, transfusion-associated circulatory overload (TACO) in 7/5000 patients and allergic reactions in 3/5000 patients. No significant adverse events were reported in the other studies. One patient in Pei et al.[24] had a severe allergic reaction to CP and could not continue therapy.
Efficacy outcomes
We found 12 case series and 1 RCT that reported efficacy outcomes. Included amongst these 12 studies were a total of 367 patients. All patients except one had severe or critical illness [Table - 1]. Most series (11/12) showed significant benefit with the use of CP.
In the small case series by Zeng et al.,[22] 5 of 6 patients who received CP died. In this study, all 6 treated patients had bilateral pneumonia and respiratory failure and 5 of them had acute respiratory distress syndrome (ARDS). All these patients were placed on mechanical ventilation and 3 required extracorporeal membrane oxygenation (ECMO). In addition, 3 of 6 patients had septic shock and 3 required dialysis. Five patients were given IVIG, 4 antivirals and 4 steroids without any improvement. The median dose of CP was 300 ml and the antibody titre in donor plasma was not mentioned. The mean duration of illness was 45.5 (37.8–59) days before CP was administered. Despite negative results, all 6 patients cleared the virus after therapy with CP. In this study, 14 of 15 control patients died.
In a large observational study of 138 patients with 1430 controls, the authors reported a 50% reduction in mortality with CP. However, the overall mortality was low in both the treated and control groups (2.2% and 4.1%, respectively) despite all the patients having severe or critical illness.[28]
The results of the RCT by Li et al.[15] showed no difference between the treated and control groups with regard to the primary outcome, i.e. clinical improvement (51.9% with the CP v. 43.1% with standard care (mean difference 8.8% [95% CI -10.4% to 28.0%]; hazard ratio [HR] 1.40 [95% CI 0.79–2.49]; p=0.26) among all patients. However, in a subgroup analysis of patients with severe disease, clinical improvement occurred in 91.3% (21/23) in the CP group v. 68.2% (15/22) in the control group (HR 2.15 [95% CI 1.07–4.32]; p=0.03). Treatment with CP led to a higher rate of viral reverse transcriptase-polymerase chain reaction (RT-PCR) becoming negative (suggestive of viral clearance) at 72 hours in 87.2% in the CP group v. 37.5% in the control group (OR 11.39 [95% CI 3.91–33.18]; p<0.001). There was no difference in secondary outcomes. Only 2/103 patients in the CP group experienced adverse events within hours after transfusion, which improved with supportive care.
Critical appraisal
There was heterogeneity among various studies on multiple factors. First, there was some variability in the inclusion criteria; second, presence of comorbid conditions such as renal failure, chronic obstructive pulmonary disease (COPD) and pregnancy among treated patients; third, variability in dose of CP from 200 to 2400 ml; and finally, the timing of intervention, which was given at different time points of illness. Not all studies reported antibody titres or neutralization assays. The outcome assessments were non-blinded in all studies. The majority of patients were on multiple concomitant therapies including steroids, antiviral agents, Chinese herbal medicines and IVIG.
[Table - 2] gives a summary of the JBI critical appraisal. Only five case series had controls and of those only two were explicitly matched controls. Despite these limitations, the effect size was high since majority of patients improved in 11 of the 12 case series. There were many possible reasons for the negative outcome in the only trial that did not show benefit. These include enrolment of extremely sick patients with multi-organ failure, late administration and probably suboptimal dosing of CP. Being an RCT, the study by Li et al.[15] provides credible evidence. The limitations of this RCT include premature termination, relatively small sample size for subgroup analysis of patients with severe disease, non-use of normal plasma as placebo, and considerable delay from onset to randomization (median 30 days). Thus, the study showed that CP was unlikely to benefit patients with life-threatening disease late in the clinical course.
Quality of evidence
As most were observational studies, the initial assignment of level of quality for the body of evidence was ‘low’ according to GRADE. It was downgraded to ‘very low’ as there was substantial heterogeneity and the sample size of the studies individually as well as collectively was small, and hence associated with very serious imprecision of point estimates. Although 11 of 12 case series suggested benefit, the only RCT available did not show benefit. There was no demonstration of a dose–response gradient in any study.
Discussion
The Covid-19 pandemic and lack of therapeutic options have made us revisit an age-old option of CP therapy. This systematic review shows that good quality evidence from multiple RCTs supporting the intervention with CP is lacking. The reported cohort studies with all their limitations suggest that use of CP in Covid-19 patients is feasible and probably safe. The large study on 5000 patients has so far published only safety data[18] and it showed that transfusion-related complications are very rare—transfusion-associated circulatory overload (0.14%, 0.07%–0.29%), TRALI (0.22%, 0.12%–0.39%) and severe allergic transfusion reaction in only 0.06% (0.02%–0.18%) patients. This is less than results reported following transfusion of fresh frozen plasma and other blood component use in critically ill ICU patients.[30],[31]
Severe allergic reactions can occur as CP is a biological product, though increased chances of serious reactions are more likely in patients with IgA deficiency or prior allergy to plasma products. Strict monitoring during infusion is mandatory and haemovigliance reporting should be done for all patients to better underst and why some patients develop complications.
With regard to efficacy, the effect size was large in 11 of 12 non-randomized studies, which reported efficacy. This may be questionable as discussed in the critical appraisal and subject to high-risk of bias including publication bias of only positive case series. The RCT by Li et al.[15] failed to show overall reduction in mortality but showed that there was a possibility that CP therapy might be effective in severe cases. The authors discussed that because the test for interaction by disease severity was not statistically significant, the findings for the severe and life-threatening subgroups should not be interpreted as different. However, as acknowledged by the authors it was possible that the study was underpowered due to early termination to detect a statistically significant difference. It is now becoming clearer that those with life-threatening illness are less likely to benefit from CP.[15],[25] One of the reasons for less than expected benefit could be late administration with the median time to randomization being 30 days. Even a small benefit in elderly patients (median age 70 years) is important in the absence of any other effective therapy. The accompanying editorial for the RCT provides historical and biological justification and shows optimism about possibilities with CP therapy for patients with severe Covid-19.[32]
Conclusion
This systematic review uncovered several important gaps which need to be addressed regarding the efficacy of CP for Covid-19. Well-designed RCTs with blinded assessment and longer follow-up are required to test the hypothesis that CP can help patients with Covid-19. US FDA,[33] and European agency,[34]
DCGI, India have approved clinical trials for CP therapy and many trials are ongoing to fill the present gaps in our knowledge.
| 1. | Casadevall A, Scharff MD. Return to the past: The case for antibody-based therapies in infectious diseases. Clin Infect Dis 1995;21:150-61. [Google Scholar] |
| 2. | Robbins JB, Schneerson R, Szu SC. Perspective—Hypothesis: Serum IgG antibody is sufficient to confer protection against infectious diseases by inactivating the inoculum. J Infect Dis 1995;171:1387-98. [Google Scholar] |
| 3. | Casadevall A, Pirofski L. Antibody-mediated regulation of cellular immunity and the inflammatory response. Trends Immunol 2003;24:474-8. [Google Scholar] |
| 4. | Zhou B, Zhong N, Guan Y. Treatment with convalescent plasma for influenza A (H5N1) infection. N Engl J Med 2007;357:1450-1. [Google Scholar] |
| 5. | Wu X-X, Gao H-N, Wu H-B, Peng X-M, Ou H-L, Li L-J. Successful treatment of avian-origin influenza A (H7N9) infection using convalescent plasma. Int J Infect Dis 2015;41:3-5. [Google Scholar] |
| 6. | Arabi YM, Haj eer AH, Luke T, Raviprakash K, Balkhy H, Johani S, et al. Feasibility of using convalescent plasma immunotherapy for MERS-CoV infection, Saudi Arabia. Emerg Infect Dis 2016;22:1554-61. [Google Scholar] |
| 7. | Zhang J-S, Chen J-T, Liu Y-X, Zhang Z-S, Gao H, Liu Y, et al. A serological survey on neutralizing antibody titer of SARS convalescent sera. J Med Virol 2005; 77:147-50. [Google Scholar] |
| 8. | Cheng Y, Wong R, Soo YOY, Wong WS, Lee CK, Ng MHL, et al. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis 2005;24:44-6. [Google Scholar] |
| 9. | Yeh K-M, Chiueh T-S, Siu LK, Lin J-C, Chan PKS, Peng M-Y, et al. Experience of using convalescent plasma for severe acute respiratory syndrome among healthcare workers in a Taiwan hospital. J Antimicrob Chemother 2005;56:919-22. [Google Scholar] |
| 10. | Mair-Jenkins J, Saavedra-Campos M, Baillie JK, Cleary P, Khaw F-M, Lim WS, et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: A systematic review and exploratory meta-analysis. J Infect Dis 2015;211:80-90. [Google Scholar] |
| 11. | Casadevall A, Pirofski L-A. The convalescent sera option for containing COVID- 19. J Clin Invest 2020;130:1545-8. [Google Scholar] |
| 12. | Diagnosis and treatment protocol for novel coronavirus pneumonia (Trial version 6, revised). Available at www.kankyokansen.org/uploads/uploads/files/jsipc/ protocol_V6.pdf (accessed on 3 Jan 2020). [Google Scholar] |
| 13. | Critical appraisal tools. Checklist for case series. Available at https://joanna briggs.org/critical-appraisal-tools (accessed on 16 May 2020). [Google Scholar] |
| 14. | Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6. [Google Scholar] |
| 15. | Li L, Zhang W, Hu Y, Tong X, Zheng S, Yang J, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: A randomized clinical trial. JAMA 2020 Jun 3. Available at https:// jamanetwork.com/journals/jama/fullarticle/2766943 (accessed on 20 Jun 2020). [Google Scholar] |
| 16. | Ahn JY, Sohn Y, Lee SH, Cho Y, Hyun JH, Baek YJ, et al. Use of convalescent plasma therapy in two COVID-19 patients with acute respiratory distress syndrome in Korea. J Korean Med Sci 2020;35:e149. [Google Scholar] |
| 17. | Duan K, Liu B, Li C, Zhang H, Yu T, Qu J, et al. The feasibility of convalescent plasma therapy in severe COVID-19 patients: A pilot study. medRxiv 2020 Mar 23;2020.03.16.20036145. (accessed on 3 Jun 2020). [Google Scholar] |
| 18. | Joyner M, Wright RS, Fairweather D, Senefeld J, Bruno K, Klassen S, et al. Early safety indicators of COVID-19 convalescent plasma in 5,000 patients. medRxiv 2020 May 14;2020.05.12.20099879. (accessed on 3 Jun 2020). [Google Scholar] |
| 19. | Salazar E, Perez KK, Ashraf M, Chen J, Castillo B, Christensen PA, et al. Treatment of COVID-19 patients with convalescent plasma in Houston, Texas. medRxiv 2020 May 13;2020.05.08.20095471. (accessed on 3 Jun 2020). [Google Scholar] |
| 20. | Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA2020;323:1582-9. doi:10.1001/jama.2020.4783 [Google Scholar] |
| 21. | Ye M, Fu D, Ren Y, Wang F, Wang D, Zhang F, et al. Treatment with convalescent plasma for COVID-19 patients in Wuhan, China. J Med Virol 2020 Apr 15;10.1002/ jmv.25882. doi: 10.1002/jmv.25882. [Google Scholar] |
| 22. | Zeng Q-L, Yu Z-J, Gou J-J, Li G-M, Ma S-H, Zhang G-F, et al. Effect of convalescent plasma therapy on viral shedding and survival in COVID-19 patients. J Infect Dis 2020;222:38-43. doi: 10.1093/infdis/jiaa228. [Google Scholar] |
| 23. | Zhang B, Liu S, Tan T, Huang W, Dong Y, Chen L, et al. Treatment with convalescent plasma for critically ill patients with SARS-CoV-2 infection. Chest 2020;158: e9-e13. doi: 10.1016/j.chest.2020.03.039. [Google Scholar] |
| 24. | Pei S, Yuan X, Zhang ZZ, Yao RR, Xie Y, Shen MM, et al. Convalescent plasma to treat COVID-19: Chinese strategy and experiences. medRxiv 2020 Apr 11;2020.04.07.20056440. (accessed on 20 Jun 2020). [Google Scholar] |
| 25. | Liu STH, Lin H-M, Baine I, Wajnberg A, Gumprecht JP, Rahman F, et al. Convalescent plasma treatment of severe COVID-19: A matched control study. medRxiv 2020 May 22;2020.05.20.20102236. (accessed on 20 Jun 2020). [Google Scholar] |
| 26. | Hegerova L, Gooley T, Sweerus KA, Maree CL, Bailey N, Bailey M, et al. Use of convalescent plasma in hospitalized patients with Covid-19—Case series. Blood 2020 Jun 19;blood.2020006964.doi: 10.1182/blood.2020006964. [Google Scholar] |
| 27. | Niu A, McDougal A, Ning B, Safa F, Luk A, Mushatt DM, et al. COVID-19 in allogeneic stem cell transplant: High false-negative probability and role of CRISPR and convalescent plasma. Bone Marrow Transplant 2020 Jun 15; 1-3. doi: 10.1038/s41409-020-0972-8. [Google Scholar] |
| 28. | Xia X, Li K, Wu L, Wang Z, Zhu M, Huang B, et al. Improved clinical symptoms and mortality on severe/critical COVID-19 patients utilizing convalescent plasma transfusion. Blood 2020 Jun 23;blood.2020007079. doi: 10.1182/blood. 2020007079. [Google Scholar] |
| 29. | Jin C, Gu J, Yuan Y, Long Q, Zhang Q, Zhou H, et al. Treatment of six COVID-19 patients with convalescent plasma. medRxiv 2020.05.21.20109512; doi: https:/ /doi.org/10.1101/2020.05.21.20109512 [Google Scholar] |
| 30. | Bosboom JJ, Klanderman RB, Migdady Y, Bolhuis B, Veelo DP, Geerts BF, et al. Transfusion-associated circulatory overload: A clinical perspective. Transfus Med Rev 2019;33:69-77. [Google Scholar] |
| 31. | Gajic O, Rana R, Winters JL, Yilmaz M, Mendez JL, Rickman OB, et al. Transfusion-related acute lung injury in the critically ill: Prospective nested case-control study. Am J Respir Crit Care Med 2007;176:886-91. [Google Scholar] |
| 32. | Casadevall A, Joyner MJ, Pirofski L-A. A randomized trial of convalescent plasma for Covid-19—potentially hopeful signals. JAMA 2020 Jun 3. doi: 10.1001/jama. 2020.10218. [Google Scholar] |
| 33. | FDA. Recommendations for investigational COVID-19 convalescent plasma. 2020 May 1. Available at www.fda.gov/vaccines-blood-biologics/investigational-new-drug-iug-or-device-exemption-ide-procers-chercrecomm.endation;ti investigational-covid-19-convalescent-plasma (accessed on 12 May 2020). [Google Scholar] |
| 34. | Draguet V. COVID-19 convalescent plasma transfusion. Public Health-European Commission. 2020. Available at https://ec.europa.cu/hcalthOblood_tieeuce_ orgaes/covi0-19_ee (accessed on 16 May 2020). [Google Scholar] |
Fulltext Views
2,569
PDF downloads
1,172




