Translate this page into:
Diabetic myonecrosis in end-stage renal disease
Correspondence to MOHIT NAREDI, mohitnaredi@gmail.com
To cite: Naredi M, Adhikari N, Bhardwaj GP, Saxena S, Bansal R, Sharma A. Diabetic myonecrosis in end-stage renal disease. Natl Med J India 2021;34:206–10.
Abstract
Diabetic myonecrosis or muscle infarction (DMI), a clinicoradiological entity is an underdiagnosed complication of diabetes mellitus. It refers to spontaneous aseptic necrosis of skeletal muscles commonly of the lower limb without evidence of any large vessel disease. It presents as painful swollen limb without any external insult in patients with long-standing diabetes mellitus with other microvascular complications especially nephropathy. We present four instances of DMI in our patients who had end-stage renal disease with a varied course.
INTRODUCTION
Diabetic muscle infarction (DMI), a clinico-radiological entity is a rare complication of diabetes mellitus. Spontaneous aseptic necrosis of the skeletal muscles usually of the lower limb occurs without evidence of any large vessel disease. It presents as a painful swollen limb in patients with long-standing diabetes with other microvascular complications. We present four patients with end-stage renal disease (ESRD) and DMI who had a varied course.
THE CASES
Case 1
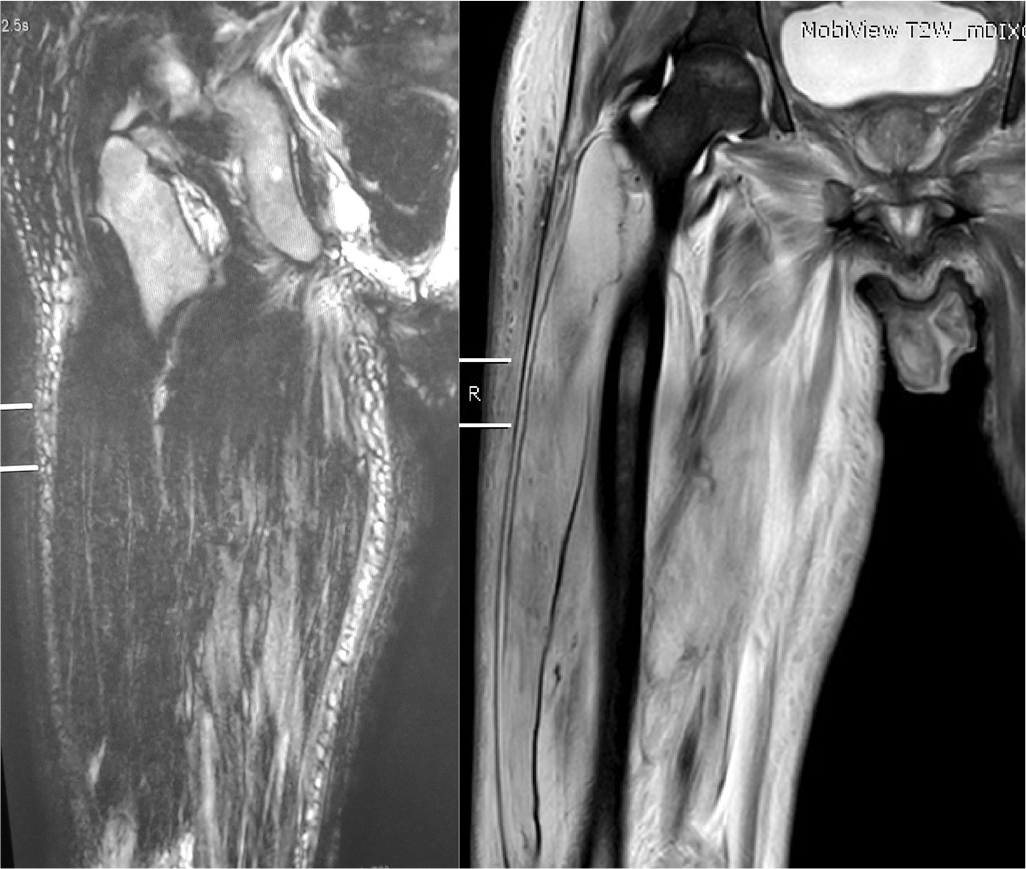
A 54-year-old woman presented with pain and swelling in the right thigh for 2 days. She had type 2 diabetes for 14 years, with retinopathy and nephropathy leading to ESRD and was on maintenance haemodialysis for one month. On examination, there was swelling involving the right thigh, more on the lateral aspect, with no warmth or redness and was tender to palpation. Movement across the right hip and knee was painful but the range of motion was not reduced and she could walk with support. The rest of the limb was normal. Her total leucocyte count was 12 690/cmm with polymorphonuclear leucocytes 80%, erythrocyte sedimentation rate (ESR) 52 mm/1st hour, C-reactive protein (CRP) 118, creatinine phosphokinase (CPK) 196 i.u./L (normal 0–170) and HbA1c 7.8 g/dl. A duplex ultrasound scan of the thigh excluded deep or superficial venous thrombosis. Magnetic resonance imaging (MRI) of the thigh showed hypointensity in the right vastus lateralis on T1-weighted imaging and hyperintensity on short tau inversion recovery (STIR)-weighted imaging with diffuse subcutaneous oedema and preserved muscle architecture (Fig. 1). The patient was managed with rest to the limb and oral paracetamol and tramadol along with glycaemic control and regular haemodialysis. The patient improved in 2 weeks and was symptom-free in 4 weeks.

- Magnetic resonance imaging, T1-weighted showing hypointensity in right vastus lateralis (right panel) and hyperintensity on short tau inversion recovery-weighted imaging (left panel) with diffuse subcutaneous oedema and preserved muscle architecture (case 1)
Case 2
A 53-year-old man presented with complaints of pain and swelling in the right thigh for 3 weeks along with difficulty in walking. The patient had type 2 diabetes for 13 years with retinopathy, neuropathy, hypertension and nephropathy. He was on maintenance haemodialysis for 2 years. On examination, there was swelling and tenderness in the right thigh along with pitting oedema and a superficial ulcer in the right foot. The movement in the right hip and knee were painfully restricted and the patient did not tolerate passive movements to the right lower limb. His total leucocyte count was 8850/cmm with 82% polymorphonuclear leucocytes, ESR 39 mm/1st hour, CRP 121 mg/L, CPK 108.40 i.u./L and HbA1c was 6.5 g/dl. X-ray of the right hip and thigh did not show any bony abnormality. Venous Doppler did not suggest any thrombosis.
MRI revealed extensive signal changes in the anterior compartment of the right thigh along with mild oedema and atrophy of rest of the muscles of the right thigh (Fig. 2). Cultures showed the ulcer was sterile. The patient was managed with limb rest, transdermal buprenorphine for pain and regular dressing of right foot ulcer along with regular haemodialysis and nutritional support. The patient showed clinical improvement and was discharged. At 2 months of follow-up, the patient was still having pain and swelling in his right thigh but could ambulate with the help of a stick and was on oral paracetamol ad libitum.
- T1- and T2-weighted (left and right panel) magnetic resonance imaging showing signal changes in the anterior compartment of right thigh along with mild oedema and atrophy of rest of the muscles of the right thigh (case 2)
Case 3
A 48-year-old woman presented with complaints of progressive pain in her right thigh for the past 1 month. She had type 2 diabetes for the past 10 years with triopathy and was on maintenance haemodialysis for the past 2 months. Pain was intermittent, increased during the past 7 days and was not able to ambulate herself. On examination, her right thigh was swollen, firm and tender, more on the medial aspect, local temperature was normal with painful restriction of movement in her right knee and hip. Her total leucocyte count was 8390/cmm with 85% polymorphonuclear leucocytes, ESR 43, CRP 93 mg/L, CPK 174.80 i.u./L and HbA1c 5.9 g/dl. Her Doppler was negative for any signs of venous involvement and MRI showed diffuse hyperintense signal changes in STIR-weighted images in muscles of the anterior and medial compartment of the right thigh along with subcutaneous oedema (Fig. 3).
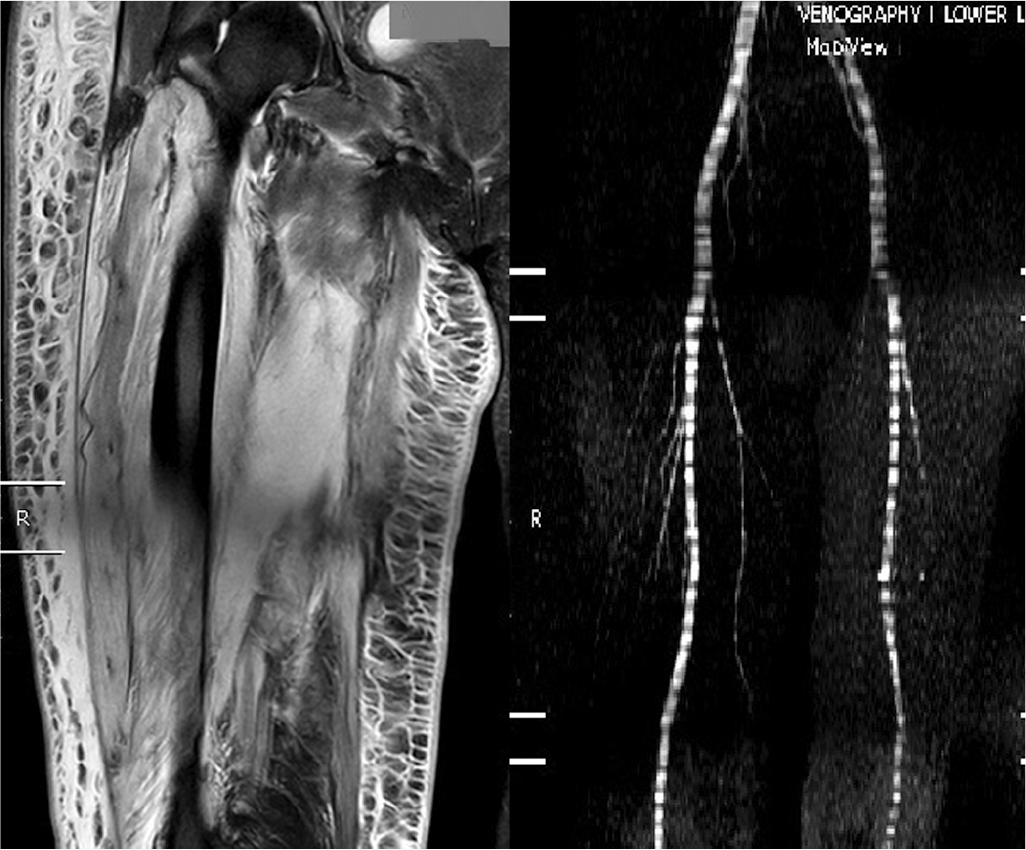
- Magnetic resonance imaging of diffuse hyperintense signal changes in short tau inversion recovery-weighted images in muscles of anterior and medial compartment of right thigh along with subcutaneous oedema. Magnetic resonance venography showing the patent venous system (right panel, case 3)
The patient was managed with transdermal buprenorphine for pain along with rest and regular haemodialysis. She responded well to this treatment and was discharged after a week. On follow-up visits, the patient was not able to walk unsupported because of pain.
Case 4
A 65-year-old man who had type 2 diabetes and hypertension for 8 years with retinopathy and nephropathy for 4 years presented to the hospital with pain in the right hip for 1 week and fever for 3 days. On presentation, the patient was febrile (100 °F) with swelling in the right gluteal region, which was tender and firm on palpation without warmth or erythema. Movements in the right hip were painful both actively and passively, and the patient was not able to walk without support. Investigations showed a total leucocyte count of 5370/cmm with 72% neutrophils, ESR 66 mm/hour, CRP 177.7 mg/L, creatinine 3.80 mg/dl, HbA1c 10.2 g/dl and CPK was 175 i.u./ml. Blood culture was sterile. Doppler of the lower limbs did not suggest venous thrombosis. X-ray pelvis was normal. MRI revealed hypointense changes in T1-weighted images in the right gluteal muscles, extensive hyperintense signal changes in STIR-weighted images along with subcutaneous oedema, without any signs of intramuscular collection (Fig. 4).
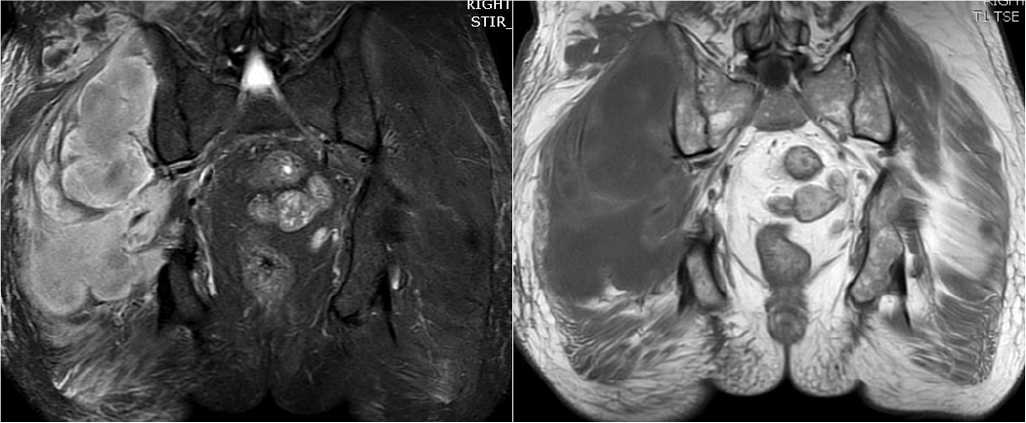
- Magnetic resonance imaging showing hypointense changes in T1-weighted images (left panel) in right gluteal muscles, extensive hyperintense signal changes in short tau inversion recovery-weighted images (right panel) along with subcutaneous oedema, without any signs of intramuscular collection (case 4)
The patient was managed conservatively with rest, injectable paracetamol and transdermal buprenorphine. The patient showed symptomatic improvement and was discharged. One month later, the patient again reported to the emergency department with confusion and high-grade fever. He was disoriented and was admitted to the intensive care unit. His total leucocyte count was 7500/cmm with 75% neutrophils and ESR 110. Serial procalcitonin levels showed a rising trend. Ultrasound and MRI pelvis showed muscle oedema without any collection. The patient was managed with fluid resuscitation, intravenous antibiotics and inotropic support but his condition deteriorated and required ventilatory support and high-dose vasopressors. He developed shock, severe metabolic acidosis, his sensorium worsened and he became anuric and required renal replacement therapy in the form of continuous venovenous haemodiafiltration. He died of sepsis on the next day (Table I).
| Characteristic | Case 1 | Case 2 | Case 3 | Case 4 | Summary statistics (mean/%/n) |
|---|---|---|---|---|---|
| Age (years) | 54 | 53 | 48 | 65 | 55 |
| Gender | Female | Male | Female | Male | 1:1 |
| Diabetes type 1/2 | 2 | 2 | 2 | 2 | All type 2 |
| Duration of diabetes (years) | 14 | 13 | 10 | 8 | 11.3 |
| Mean HbA1c | 7.8 | 6.5 | 5.9 | 10.2 | 7.6 |
| Nephropathy | Present | Present | Present | Present | All |
| Neuropathy | Absent | Absent | Present | Present | Half |
| Retinopathy | Absent | Present | Present | Present | Half |
| Dialysis modality | HD | HD | HD | None | 3 of 4 |
| Months on dialysis | 1 | 24 | 2 | 0 | 9 |
| Muscles (index) | |||||
| Thigh | + | + | + | - | 75% |
| Glutei | - | - | - | + | 25% |
| Clinical features | |||||
| Pain | Present | Present | Present | Present | 100% |
| Fever | Absent | Absent | Absent | Present | 25% |
| Recurrence | Absent | Absent | Absent | present | 25% |
| Laboratory studies | |||||
| Leucocytosis | 12.69 | 8.89 | 8.39 | 5.37 | 8.835 |
| CPK | 196 | 108.4 | 174.8 | 175 | 163.55 |
| ESR | 52 | 39 | 43 | 66 | 50 |
| CRP | 118 | 121 | 93 | 177.7 | 110.6 |
| Cultures | Sterile | Sterile | Sterile | Staphylococcus aureus | |
| Imaging studies | |||||
| Duplex venography | Normal | Normal | Normal | Normal | 0 |
| CT scan/X-ray | Normal | Normal | Normal | Normal | 0 |
| Magnetic resonance imaging | |||||
| T1 hypointensity | Present | Present | Present | Present | 100 |
| STIR hyperintensity | Present | Present | Present | Present | 100 |
| Outcome | Complete recovery | Partial recovery | Disability | Death | - |
| Recovery duration (week) | 4 | 7 | - | - | 5.5 |
HbA1c glycosylated haemoglobin HD haemodialysis CPK creatinine phosphokinase ESR erythrocyte sedimentation rate CRP c-reactive protein STIR short tau inversion recovery
DISCUSSION
The first case of diabetic myonecrosis was described as ‘tumoriform focal muscular degeneration’ in 1965 by Angervall and Stener. They reported two patients, both underwent excision of a painful swelling in the proximal part of lower limbs, which showed ischaemic muscular necrosis.1 Since then, around 200 cases have been described. It has been reported in both type 1 and type 2 diabetes. A systematic analysis of the available literature, which included 87 references with a total 170 episodes of DMI, found that 54% occurred in women, 50% had type 2 DM, mean age of presentation was 44.6 years, mean duration of diabetes at the time of DMI diagnosis was 18.9 years for T1DM and 11.0 years for T2DM. There was a preponderance of patients with other microvascular complications (65.8% had other microvascular complications) commonly renal failure (75% had nephropathy) reflecting poor glycaemic control, evident by mean HbA1c of 9.3%.2 Another study by Yong and Khow analysed 24 publications with 41 patients having DMI.3 Of these, 53.7% were women, 17 (41.5%) had type 1 diabetes, 22 (53.7%) had type 2 diabetes, mean duration of DM at the time of DMI diagnosis was 17.3 years for type 1 diabetes and 15.7 years for type 2 diabetes, 29 patients were receiving renal replacement therapy in the form of haemodialysis (60.1%), peritoneal dialysis (21%) and renal transplant (12.2%) and the average HbA1c value was 7.2%, which is high considering the effect of renal failure on insulin metabolism. In another series by Gupta et al. from an Indian centre, the mean (SD) age reported was 45.5 (7.3) years, the mean duration of diabetes was 9 years with an equal distribution of men and women and the mean (SD) HbA1c, 9.5% (0.6%) indicated poor glycaemic control at presentation with all the patients having concomitant one or more microvascular complications (Table II).4–7
| Characteristic | Yong et al.3 | Horton et al.2 | Gupta et al.4 | Our study |
|---|---|---|---|---|
| Age (years) | 44.2 | 44.6 | 45.5 | 55 |
| Gender | Males<females | Males<females | Males<females | Males<females |
| Diabetes type 1 or 2 | Both | Both | Both | 2 |
| Duration of diabetes (years) | 17.3 | 18.9 | 9 | 11.3 |
| Mean HbA1c | 7.2 | 9.34 | 9.5 | 7.6 |
| Comorbid conditions (%) | ||||
| Neuropathy | 46.6 | 56.1 | 100 | 50 |
| Retinopathy | 65.8 | 58.5 | 100 | 75 |
| Dialysis | ||||
| Modality (HD/PD/KTP) | 25/9/5 | na | 0 | 3/0/0 |
| Months on dialysis | 25.2 | na | 0 | 9 |
| Muscles (index) | ||||
| Thigh (%) | 71.2 | 66.7/58.5 | 75 | 75 |
| Distal lower limb | 15.3 | 16.7/14.6 | 25 | 0 |
| Upper limb | 5.4 | 12.2 | 0 | 0 |
| Clinical features (%) | ||||
| Pain | 100 | 100 | 100 | 100 |
| Fever | 11 | 14.6 | 50 | 25 |
| Recurrence | 34.9 | 43.9 | 25 | 25 |
| Laboratory studies | ||||
| Leucocytosis | 42.5 | 31.4 | 50 | 25 |
| Raised CPK | 50 | 31.6 | 25 | 75 |
| Raised ESR | 83.3 | 88.9 | 50 | 50 |
| Raised CRP | 90 | 81.8 | - | 100 |
| Imaging studies (%) | ||||
| Negative duplex venography | 98.8 | 100 | 100 | 100 |
| Magnetic resonance imaging | ||||
| T1 hypointensity | Present | Present | Present | Present |
| STIR hyperintensity | Present | Present | Present | Present |
| Recovery duration (weeks) | 4.3 | 6 | 5.75 | 5.5 |
HbA1c glycosylated haemoglobin HD haemodialysis PD peritoneal dialysis KTP kidney transplant CPK creatinine phosphokinase ESR erythrocyte sedimentation rate CRP c-reactive protein STIR short tau inversion recovery
It being a rare disorder, the pathophysiology of this entity is unclear. Various hypotheses have postulated a complex interplay of atherosclerosis, microangiopathy, coagulopathy and ischaemia reperfusion injury.8 It commonly affects muscles of the lower limbs than of the upper limb and the more proximal ones, presenting as a painful swelling of the affected muscle group, which is tender and firm on palpation. There may be severe painful spasm mimicking bone fractures. There may be associated fever but systemic signs are usually uncommon and if present, point towards an infective aetiology. It is different from acute macrovascular limb ischaemia, which commonly affects the distal part of the lower limbs and is frequently limb-threatening and requires surgical treatment. The differential diagnoses include venous thrombosis—superficial and deep, infective disorders such as cellulitis, fasciitis, pyomyositis, panniculitis, osteomyelitis and septic arthritis, abscess, granulomatous lesions such as tuberculosis, sarcoid and toxoplasma, also myositis ossificans, traumatic muscle rupture, muscle haemorrhage, soft-tissue neoplasm (lymphoma/sarcoma) and calciphylaxis. Routine laboratory tests are seldom useful for the diagnosis of DMI.9 Total leucocyte count may be high with neutrophilia. CPK is elevated in <50% of patients reflecting the nature of the injury. Presumably, the onset of symptoms start after the active muscle injury is over. Inflammatory markers CRP and ESR are elevated in most of the cases. Imaging studies are the cornerstone of diagnosis. Ultrasound Doppler is helpful to rule out deep vein thrombosis. Skiagram and CT scan may be done to rule out bony pathology. Diagnosis is made on MRI, which on T1-weighted imaging shows hypointensity of the affected muscle group; diffuse enlargement of involved muscle groups and minimal loss of normal fatty intermuscular septa; focal or diffuse muscle, subcutaneous and fascial plane oedema; no obvious filling defect and increased signal intensity on STIR-weighted images superimposed on an otherwise normal appearance of the adjacent muscles (Table III). Muscle oedema can be subtle and detectable only with inversion-recovery or fat-suppressed T2-weighted images. On contrast administration, the affected muscle shows <10% enhancement suggesting the ischaemic nature of the disease. The sensitivity of STIR-MRI ranges from 78% to 100% but specificity is 40%–50% for muscle infarction.10 The most commonly affected muscles reported are the thigh muscles: vastus medialis, followed by vastus lateralis, the vastus intermedius, the rectus femoris, the soleus and the gastrocnemius.11 Almost all cases reported involved limb muscles, and no case involving axial skeletal musculature was reported. Biopsy is not routinely required for management nor is recommended as it only increases the morbidity without much help in diagnosis. In few cases, which reported gross histopathology, the muscle was pale and swollen. Microscopic examination shows microangiopathy, areas of microinfarction and inflammatory infiltrate and areas of necrosis. In later stages, areas of muscle regeneration or fibrosis can be seen (Fig. 5). Treatment is largely supportive with spontaneous recovery within few weeks being the usual outcome. Pain can be managed with non-steroidal anti-inflammatory drugs, opioids and rest to the affected limb. Recurrences are common, which make overall long-term outcome poor, leading to poor glycaemic control and end-organ damage.12
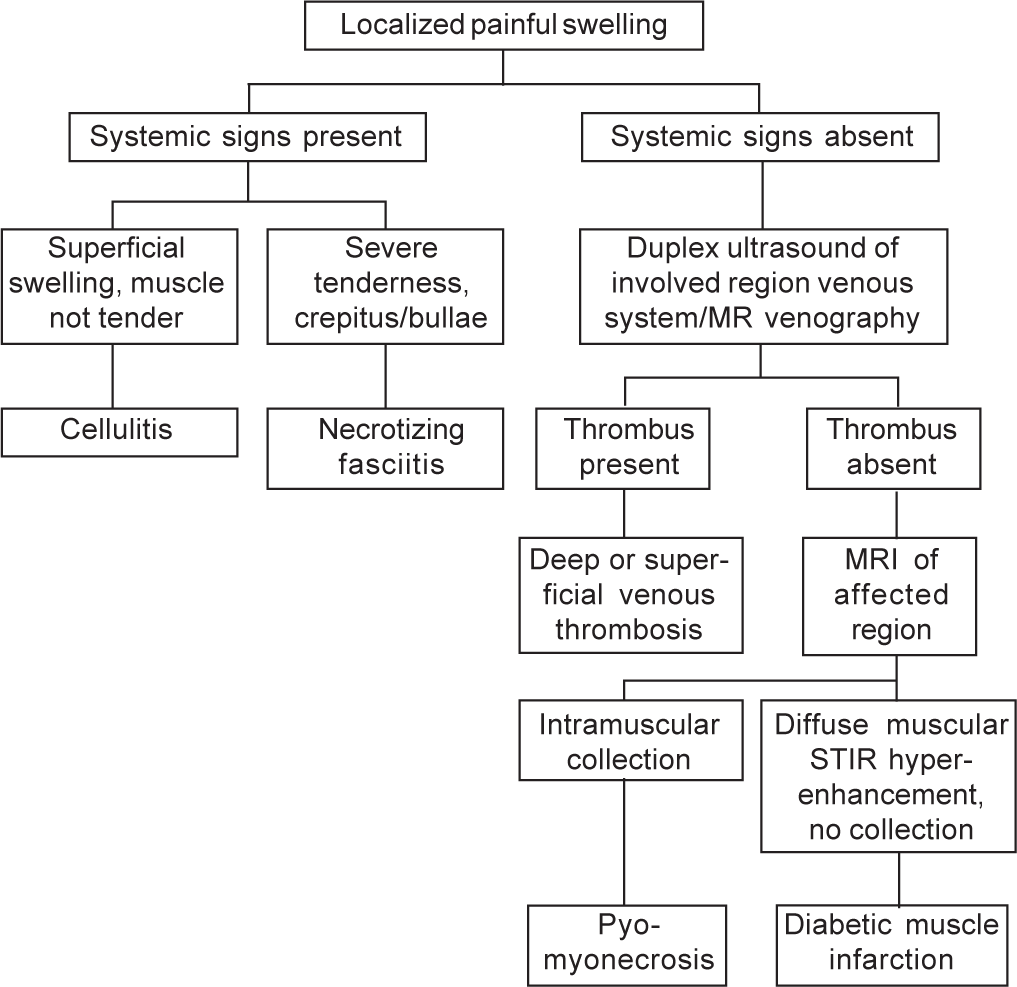
- Diagnostic algorithm MR magnetic resonance MRI magnetic resonance imaging STIR short tau inversion recovery
| Diagnosis | T1, T2, STIR, FLAIR |
|---|---|
| Myonecrosis | On T1 muscles are hypo- or isointense suggestive of muscle ischaemia or necrosis, on STIR heterogeneous bright signal seen suggestive of oedema in affected muscle and surrounding areas; muscle architecture is preserved, fat suppression and subtraction of contrast-enhanced images is helpful |
| Necrotizing fasciitis/cellulitis | Fluid is seen in interfacial plane with fascial thickening (≥3 mm) and hyperenhancement. Gas may be seen along the fascial planes |
| Pyomyositis | Localized smooth-walled intramuscular collection suggestive of abscess, demonstrates low T1 and hyper T2 signal and rim enhancement with central non-enhancement |
| Venous thrombosis | Oedema of deep muscle and fascia on images obtained with fluid-sensitive sequences, magnetic resonance venography of affected region can show up the thrombus |
| Osteomyelitis | Bone marrow oedema immediately adjacent to a soft-tissue infection or ulcer, with or without evidence of cortical destruction, hyperintense marked associated periosteal oedema |
| Neuropathic arthropathy | Extensive soft-tissue oedema occurring in the absence of infection or ulceration, multiple foci of marrow oedema, prominent subchondral oedema and enhancement may extend far into the medullary cavity, although superimposed fractures also can contribute to changes in marrow signal intensity |
| Soft-tissue mass | Uniform high signal intensity on T1-weighted images suggests the presence of the blood breakdown product met Hb, proteinaceous material, fat. For example, intramuscular haematomas and myositis ossificans may demonstrate peripheral calcification on radiographs |
STIR short tau inversion recovery FLAIR fluid attenuation inversion recovery Hb haemoglobin
Conclusion
DMI is an uncommon complication seen in patients with longstanding diabetes with nephropathy and should be suspected when such patients develop localized lower limb muscle pain and swelling without systemic signs. Closest differentials are venous thromboses and necrotizing fasciitis and a thorough clinical examination with duplex ultrasound and T2-weighted MRI scan is sufficient to ascertain or rule out the diagnosis. It should be managed with supportive care without antibiotics or anticoagulation. Prompt diagnosis obviates the need for invasive procedures. More awareness and better utilization of the available diagnostic modalities such as MRI will lead to early detection. Systematic studies are needed to understand the natural history of disease and possible treatment.
Conflicts of interest
None declared
References
- Tumoriform focal muscular degeneration in two diabetic patients. Diabetologia. 1965;1:39-42.
- [CrossRef] [Google Scholar]
- Diabetic muscle infarction: A systematic review. BMJ Open Diabetes Res Care. 2015;3:e000082.
- [CrossRef] [PubMed] [Google Scholar]
- Diabetic muscle infarction in end-stage renal disease: A scoping review on epidemiology, diagnosis and treatment. World J Nephrol. 2018;7:58-64.
- [CrossRef] [PubMed] [Google Scholar]
- Clinico-radiological characteristics and not laboratory markers are useful in diagnosing diabetic myonecrosis in Asian Indian patients with type 2 diabetes mellitus: A 10-year experience from South India. J Family Med Prim Care. 2018;7:1243-7.
- [CrossRef] [PubMed] [Google Scholar]
- Diabetic myonecrosis: An Indian experience. Clin Diabetes. 2013;31:53-8.
- [CrossRef] [Google Scholar]
- Spontaneous diabetic myonecrosis: Report of four cases from a tertiary care institute. Endocrinol Diabetes Metab Case Rep. 2015;2015:150003.
- [CrossRef] [PubMed] [Google Scholar]
- Diabetic myonecrosis: An underreported complication of diabetes mellitus. Indian J Endocrinol Metab. 2011;15:S58-61.
- [CrossRef] [PubMed] [Google Scholar]
- Diabetic musculoskeletal complications and their imaging mimics. Radiographics. 2012;32:1959-74.
- [CrossRef] [PubMed] [Google Scholar]
- Lower limb complications of diabetes mellitus: A comprehensive review with clinicopathological insights from a dedicated high-risk diabetic foot multidisciplinary team. Br J Radiol. 2015;88:20150135.
- [CrossRef] [PubMed] [Google Scholar]
- Magnetic resonance imaging of painful swollen legs in the emergency department: A pictorial essay. Emerg Radiol. 2017;24:577-84.
- [CrossRef] [PubMed] [Google Scholar]
- Diabetic muscle infarction: An underdiagnosed complication of long-standing diabetes. Diabetes Care. 2003;26:211-15.
- [CrossRef] [PubMed] [Google Scholar]




