Translate this page into:
Economic burden of beta-thalassaemia major receiving hypertransfusion therapy at a public hospital in Mumbai
Correspondence to MAMTA MURANJAN; muranjanmamta@rediffmail.com
[To cite: Uchil A, Muranjan M, Gogtay NJ. Economic burden of beta-thalassaemia major receiving hypertransfusion therapy at a public hospital in Mumbai. Natl Med J India 2023;36:11–16. DOI:10.25259/NMJI_580_20]
Abstract
Background
Treating beta-thalassaemia major may entail high costs with considerable out-of-pocket expenditure. Therefore, determination and valuation of the economic costs of a common haemoglobinopathy such as beta-thalassaemia major in India may provide insights to evolve policies for reduction or elimination of the disease. We estimated economic burden of beta-thalassaemia major in Mumbai in terms of cost to the family and the healthcare system.
Methods
This single-centre, prospective, cross-sectional, non-interventional study included children <12 years of age treated at the thalassaemia day care centre of a public hospital in Mumbai. The demographic data and treatment-related information was recorded. Cost of illness was studied from a societal perspective by the prevalence-based approach. Direct (medical and non-medical), indirect (loss of wages and loss of school days) and intangible costs (closed-ended iterative bidding) were calculated for each patient by interview.
Results
The total annual cost of treating 130 children with beta-thalassaemia major in Mumbai was ₹86 72 412 (US$ 127 535) or ₹66 710 (US$ 981) per patient per year and ₹12 82 30 412 (US$ 1 885 741) including intangible costs. Direct costs contributed to 94% of the cost of illness with chelation therapy (23%) and blood investigations (21%) being major contributors. Direct and indirect costs correlated significantly with duration of blood transfusion (p<0.05 and p=0.006, respectively), whereas indirect costs correlated with socioeconomic status (rho=0.25).
Conclusion
The majority (94%) of costs incurred by families for treatment of beta-thalassaemia major are direct costs, especially expenses for chelation and blood investigations. Even at subsidized rates, financial burden to the families from lower socioeconomic strata is likely to be considerable as these are out-of-pocket expenses. In consideration of the economic impact of treating beta-thalassaemia major in individual families, the healthcare system and society, it is prudent to promote and pursue long-term and short-term measures with urgent emphasis on prevention as a public health activity at the national level in India.
INTRODUCTION
The National Health Policy (2017) recommends an increase in government expenditure on health from 1.15% of gross domestic product in 2017 to 2.5% by 2025.1 The recommendation for greater investment in healthcare strives to reduce the proportion of households facing catastrophic health expenditure by 25% by 2025.1 In India, out-of-pocket expenditure exceeds 60% of the total health expenditure, which plunges households into poverty, impoverishment and massive debts.2 Therefore, studies that undertake economic evaluations would contribute to evidence in this area and inform local and national policy. One of the means to evaluate economic burden of a disease on the society is to estimate cost of illness (COI).3 COI studies aid in the identification of a disease that requires prioritization in terms of healthcare policies and can direct the allocation of budgetary resources for policy efficiency.3
Beta-thalassaemia major is one of the most common haemoglobinopathies in India with an estimated incidence of 1 in 2700 live births.4 The cost of treating thalassaemia is high with considerable out-of-pocket expenditure. Factors that determine the economic burden of thalassaemia are birth prevalence and survival of the affected infants; availability and costs and treatment effectiveness; social support (or lack thereof) and acceptance and integration of the affected individuals in society.5 Studying the economic burden of thalassaemia serves multiple goals: it helps various stakeholders understand the burden of thalassaemia major on patients’ families,6 the cost-effectiveness of a prenatal screening programme can be assessed,7 long-term incidence-based cost of beta-thalassaemia major to healthcare services can be estimated8 and it can serve as a tool for comparison of cost in different hospitals or cost of various iron chelators.9,10
We undertook this study to estimate the economic burden of treating beta-thalassaemia major in Mumbai in terms of cost to the family and the healthcare system.
METHODS
Ethical issues
The study was approved by the institutional ethics committee. Eligible children were recruited after obtaining parents’ consent and/or child’s assent.
Study design and setting
This single-centre, prospective, cross-sectional, noninterventional study was conducted at the thalassaemia daycare centre of the department of paediatrics in a tertiary care public hospital. The thalassaemia daycare centre has 156 registered patients receiving triple saline washed packed cell transfusions every 3–4 weeks with a target pre-transfusion haemoglobin of 9 to 10 g/dl. The protocol for investigations and monitoring was as follows: haemogram at every visit; reticulocyte count, normoblast count, aspartate aminotransferase, alanine aminotransferase, blood urea nitrogen, serum creatinine and serology for HIV, hepatitis B and hepatitis C every 6 months and serum ferritin, serum calcium, serum parathormone, growth hormone, fasting blood sugar, thyroid hormone profile, electrocardiogram, two-dimensional echocardiography and Doppler, dual-energy X-ray absorptiometry scan and T2-weighted cardiac and hepatic MRI annually. Minor blood grouping was performed in a subset of patients. Iron chelation therapy (ICT) with either deferasirox (20–40 mg/kg/day) or deferiprone (50–100 mg/kg/day) was commenced when serum ferritin exceeded 1000 ng/ml. Administration of hepatitis B vaccine and/or special vaccines was individualized.
Study duration
The study was conducted over a period of 23 months from January 2015 to November 2016.
Selection criteria
Children <12 years of age with a confirmed diagnosis of beta-thalassaemia major and receiving hypertransfusion therapy were recruited in the study. Families having another child or a dependent family member with unrelated chronic disease or beta-thalassaemia were excluded from the study.
Variables recorded Demographic data (age, gender and socioeconomic status), duration of treatment (age at diagnosis and age at treatment initiation in months), duration of blood transfusions and duration of ICT in years and presence of comorbid conditions and/or complications were recorded for each patient.
Socioeconomic and occupation classification
Socioeconomic status was determined by the modified Kuppuswamy scale11 using the average Consumer Price Index for industrial workers of 261 in April 2016 (http://labourbureau.nic.in/indexes.html).
Cost calculations and perspective
COI was studied from both a hospital and a societal perspective by the prevalence-based approach. Direct, indirect and intangible costs were calculated for each patient by interviewing parents and perusing invoices or receipts. The items included for calculating cost in each patient are shown in Fig. 1. All the direct medical costs (cost for diagnosis, prenatal diagnosis, carrier testing, outpatient registration charges, blood transfusion, ICT, concomitant medications, blood investigations, radiology [sonography, echocardiography, MRI of the liver and heart], procedural costs [e.g. splenectomy], special vaccines and consumables) were at subsidized rates. Direct costs included shared costs obtained from the institution’s budget section for April 2015 to March 2016. Components of shared costs for outpatients included costs for establishment (staff salaries), contingencies, medicines, instruments, special investigations, stores, repair and maintenance. As children with thalassaemia receive transfusion on a daycare basis, an average daily outpatient expenditure of ₹231 per patient was used for calculation. We did not consider bonuses and inflation rates of healthcare workers’ salary. Though patients are not charged for blood, a cost of ₹440 was considered as cost for preparing one blood bag. The institute provides iron chelators (deferiprone and deferasirox) at no cost to the patients through the National Health Mission. Costs of iron chelators, concomitant medicines and consumables, special vaccines and consumables obtained from the hospital pharmacy were based on procurement price. Travel cost was reported as the median cost paid by patients for a year for treatment-related travel. Accommodation and meals were necessary for a few outstation patients.
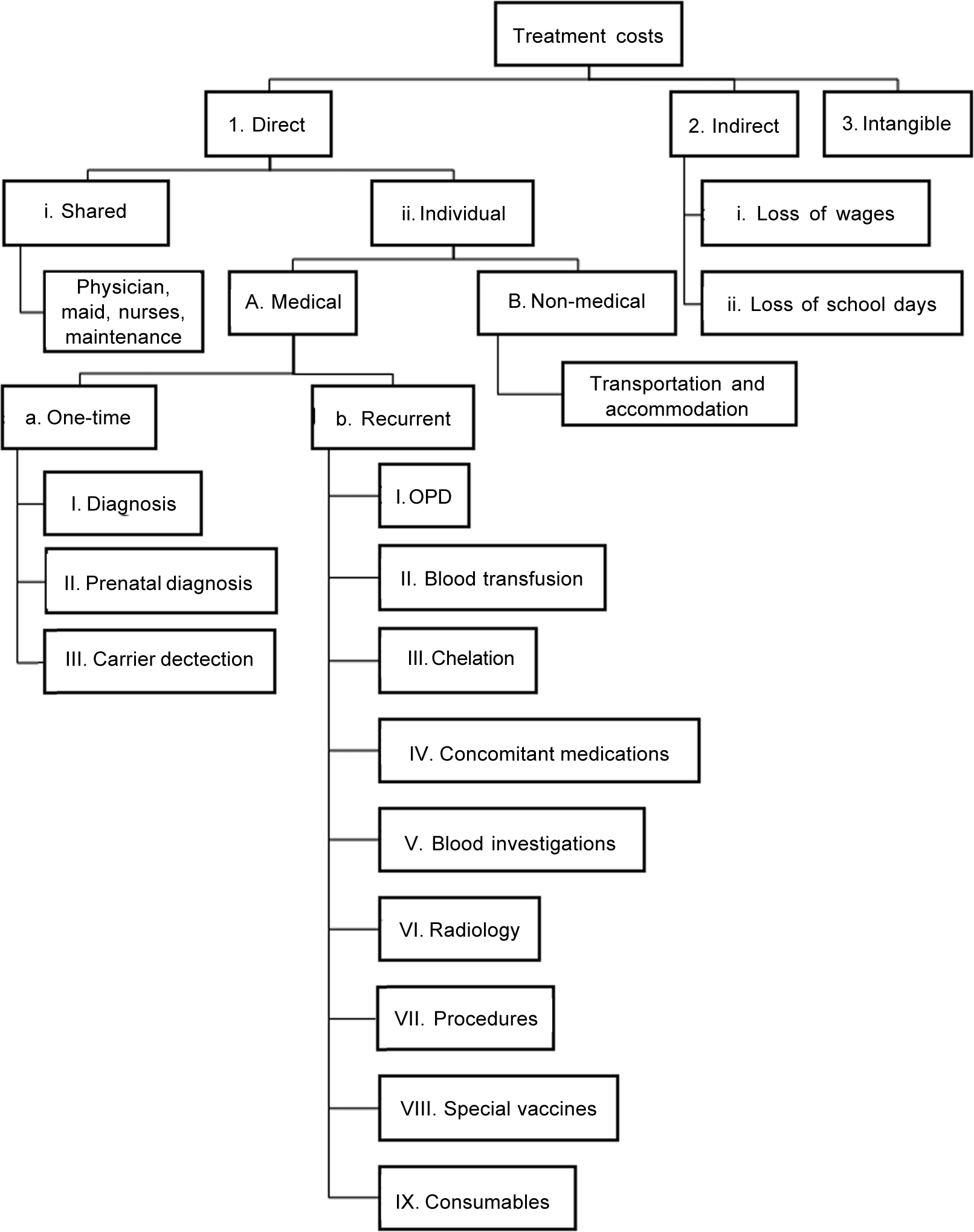
- Components considered for computing treatment costs
The human capital method was used for computing indirect costs for loss of wages (loss of income per day multiplied by the number of days lost in a year) and willingness to pay (WTP) for loss of school days.3 The current minimum wages for an unskilled worker of ₹7908 per month (www.paycheck.in/main/salary/minimumwages/maharashtra-minimum-wage-w-e-f-january-1-2016-to-june-30-2016) were considered for homemakers. This was extrapolated for 12 months to give loss of wages per year. Close-ended iterative bidding was chosen to elicit intangible costs as it can obtain specific amount of WTP. To minimize the starting bid bias, participants were given their initial bid as their respective per capita income.
Total costs (sum of direct costs and indirect costs in ₹ and $, exchange rate of 1$=₹68) (www.rbi.org.in;accessedon18Nov2016) were calculated for each patient. The measures of central tendency, mean, median and mode were calculated.
Statistical methods
Quantitative data were expressed as median (range) and qualitative data as proportions. Normality of quantitative data was assessed using the Kolmogorov–Smirnov test. The correlation between independent variables (socioeconomic status and duration of blood transfusion) and the dependent variable (direct, indirect and intangible costs) was done using the Spearman’s rank correlation coefficient. All analyses were done at 5% significance using the SPSS software version 24.
RESULTS
Demographics
Of the 156 children registered at the thalassaemia daycare centre during the study period, 130 were enrolled (6 lost to follow-up or expired, parents of 5 patients declined consent and 15 patients did not fulfil the inclusion criteria). The median age of the participants was 7 years (range 2–12 years). The M:F ratio was 1:1.2 (58 boys and 72 girls). The majority (39.2%) belonged to upper lower socioeconomic class, followed by 37.7% in lower middle class, 20% in upper middle class, 2.3% in upper class and 0.7% lower class. Age at diagnosis and treatment and nature of ICT are given in Table I. Comorbidity (Down syndrome and gastro-oesophageal reflux disease), complication (dental caries, stunting and left ventricular dysfunction) and adverse effect of blood transfusion (hepatitis C virus 1, human immunodeficiency virus 1, haemosiderosis 1 and transfusion reactions 3) were noted in our study population.
| Parameter | Median (range) |
|---|---|
| Age at diagnosis (months) | .11.6 (2–60) |
| <12 (%) | .105 (80.8) |
| >12 (%) | .25 (19.2) |
| Age at initiation of treatment (months) | 12.4 (2–60) |
| Duration of blood transfusion (years) | 5.7 (1–11) |
| Duration of iron chelation therapy (years) | 2.3 (0–9) |
| Iron chelation therapy (%) | .118 (90.7) |
| Deferasirox | .114 (96.6) |
| Deferiprone | .3 (2.5) |
| Deferasirox and deferiprone | .1 (0.9) |
| Comorbidity (%) | .11 (8.4) |
Cost analysis
Computation of direct and indirect costs is given in Table II. The median direct cost was ₹59 399 (rangè32 778–97 164) and that of indirect cost was ₹3756 (range ₹600–31 200; Figs 2a and b). For determining intangible costs, parents were asked ‘what would be their WTP for a remedy that would cure their child’s disease?’ Responses elicited from 126 parents stating a maximum amount based on the per capita income doubling bids was a median of ₹200 000 (range ₹8000–10 000 000; Fig. 2c). A significant correlation was found between the duration of blood transfusion and direct costs (p<0.05) and indirect costs (p=0.006). There was no correlation between the duration of blood transfusion and intangible costs (Table III). Correlation between socioeconomic status and the direct and intangible costs was insignificant (Table III). However, there was a good correlation (rho=0.25) between indirect costs and socioeconomic status.
| Parameter | n | Amount in ₹ (%) |
|---|---|---|
| Direct costs ₹8 047 588 | ||
| Shared costs | 130 | 360 360 (4) |
| Diagnosis | 130 | 93 260 (1) |
| Prenatal diagnosis | 16 | 153 700 (1) |
| Carrier testing | 123 | 158 100 (1) |
| Outpatient department | 130 | 15 600 (0.1) |
| Blood transfusion | 130 | 888 250 (10) |
| Iron chelation therapy | 118 | 2 011 580 (23.2) |
| Concomitant medications | 130 | 1 169 083 (13) |
| Blood investigations | 130 | 1 754 160 (21) |
| Radiology | 107 | 122 960 (1) |
| Procedures (dental extraction, splenectomy) | 2 | 56 500 (0.6) |
| Vaccines | 19 | 61 805 (0.7) |
| Consumables | 130 | 780 000 (9) |
| Transportation and accommodation | 125 | 422 240 (4) |
| Indirect costs ₹624 824 | ||
| Loss of wages | 130 | 480 924 (5) |
| Loss of school days | 9 3 | 143 900 (1) |
| Intangible costs ₹119 558 000 | 126 | – |
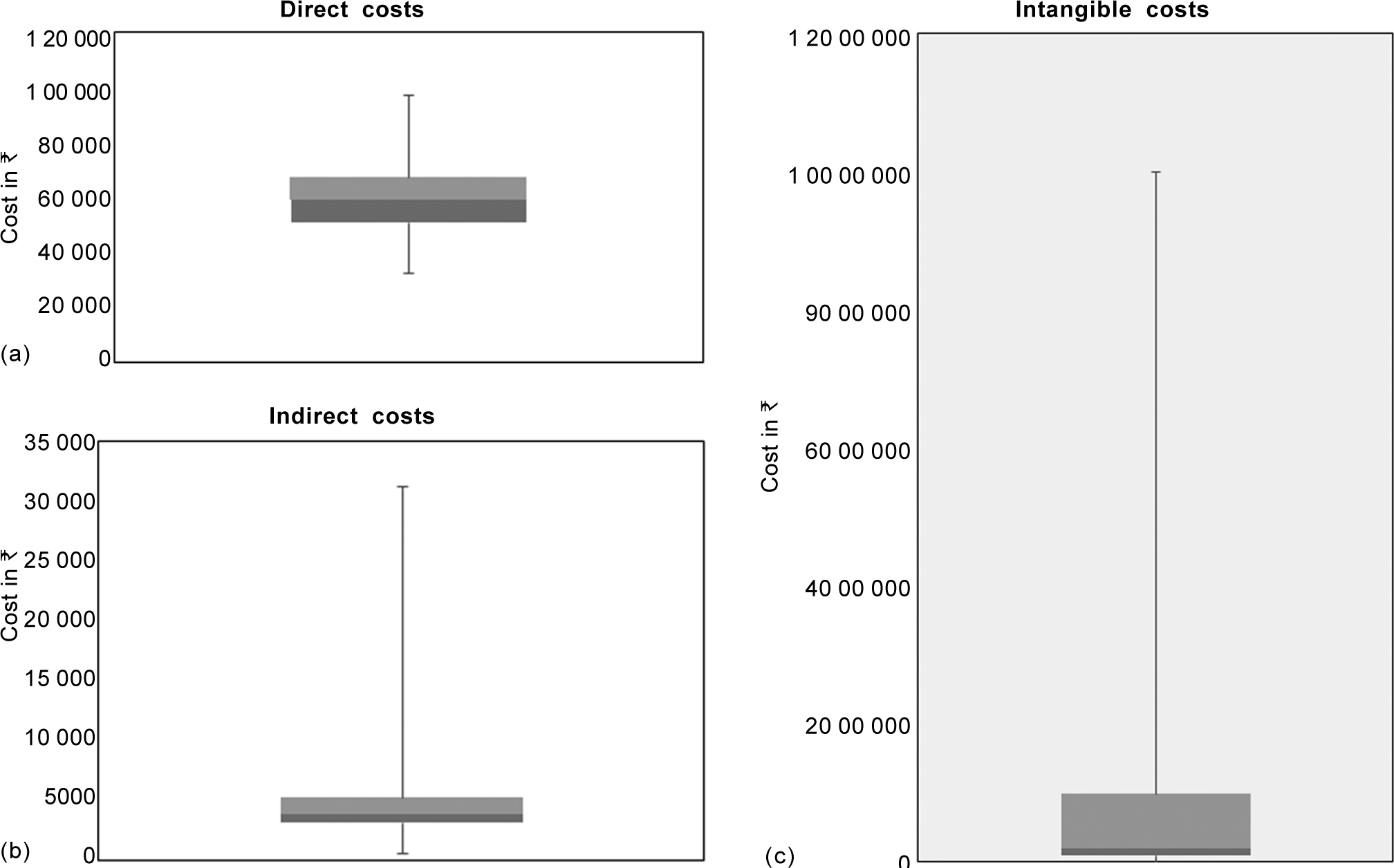
- Box and whisker plot showing median, interquartile and minimum and maximum values, (a) direct costs, (b) indirect costs and (c) intangible costs
| Parameter | Duration of blood transfusion | Socioeconomic status | ||
|---|---|---|---|---|
| Correlation coefficient (rho) | p value | Correlation coefficient (rho) | p value | |
| Direct costs | 0.5* | <0.05 | –0.011 | 0.89 |
| Indirect costs | 0.23* | 0 0 0 6 | 0 . 2 5 * | 0.03 |
| Total costs | 0.5* | <0.05 | 0 . 0 2 9 | 0.73 |
| Intangible costs | 0.15 | 0.07 | –0.011 | 0.89 |
The total annual cost of treating beta-thalassaemia major (130 patients) in Mumbai was found to bè86 72 412 ($127 535) or ₹66 710 ($981) per patient per year. Direct costs contributed to 94% of COI. Contribution by ICT (23%), followed by blood investigations (21%), accounted for a major proportion of the costs. After adding intangible costs (for 126 patients), the total annual cost was ₹12 82 30 412 ($1 885 741) or ₹986 387 ($14 505) per patient per year.
DISCUSSION
Economic burden of a disease provides information on the impact of the disease on household income, firm’s profits or on a country’s gross domestic product.12 It also helps to identify possible strategies to reduce the cost of disease or injury through preventive actions or therapy. In our study, economic burden of beta-thalassaemia major in Mumbai at a public healthcare institute as estimated by the annual cost of treatment was $14 505 per patient (including intangible cost). Direct costs accounted for 94% of the COI. Direct costs were out-of-pocket expenses, and ICT and investigations together accounted for 44% of the direct costs.
From a clinical or epidemiological viewpoint, beta-thalassaemia major is the most common haemoglobinopathy in India with a prevalence ranging from 1 to 1.5 lakh (0.1–0.15 million) individuals.13 A majority of the individuals with thalassaemia are now surviving up to 25–55 years of age with timely diagnosis, blood transfusion and ICT.14 The annual cost of optimum treatment with blood transfusion and ICT is considerable and estimated to bè125 000.15 Thus, the economic burden of thalassaemia needs to be studied comprehensively.
Few studies in India have attempted to analyse the economic burden of thalassaemia. These were COI studies focusing on estimating direct (medical and non-medical) costs15 or direct and indirect costs (loss of work days or school days).16,17 These previous COI studies revealed that the economic burden of treating thalassaemia varies from ₹2500 per month in an institute in Punjab in 2013 to approximately $137.9 (47.8) annually in Kolkata during 2007–2008 and $629 to $2300 annually in 2015 in an institute in Lucknow.15–17 Such studies inherently tend to underestimate the economic burden. We attempted to overcome some of these limitations by computing a monetary equivalent of loss of wages, assigning a monetary value to loss of school days using WTP approach and computing intangible costs in terms of WTP. By including intangible costs, our study revealed an annual cost of $14 505 per patient per year in 2015–2016. All the institutes where these studies were performed, including our institute, are public health institutes where all components of care are highly subsidized or free. In contrast, the financial burden on those seeking treatment in private healthcare institutes is logically expected to be significantly higher. One estimate of treatment cost in a private institute for a 4-year-old thalassaemic child was $1948–2164 annually.18 The expenditure varied from 18.5% ±14.3% of annual family income in the study by Mallik et al. to 38% of family income in the study by Moirangthem and Phadke.16,17 The wide variation in the economic burden in these studies could be due to socioeconomic differences in the study population, nature of subsidies offered in individual institutes, study methodology in terms of cost components included in the computation of COI and differences in the cost of living. The cost of living index is 24.7 in Lucknow, 24.8 in Kolkata and 29.03 in Mumbai (https://costoflivingreports/india,accessedon7Jun2020). Economic burden to the family determined in one city therefore cannot be extrapolated to other parts of India. An additional factor contributing to variation in economic burden (especially magnitude of out-of-pocket expenditure) across various states in India, could be the provision of iron chelators free of cost in some states (but not in others) such as Maharashtra, Delhi and Gujarat with an ongoing programme for thalassaemia control.17
All the studies on economic burden of thalassaemia in India, including our study, show that majority of the expenditure can be attributed to direct medical costs. In our study, 94% of the cost could be attributed to direct costs, of which 23% was cost incurred for ICT and 21% for investigations. The financial burden on households would be substantial as 77% of families in our study belonged to upper lower and lower middle class socioeconomic strata. Moirangthem and Phadke showed that 53% of expenses were on medications, and this component increased with increase in age as the dose of medications increased.17 Our study too revealed a significant correlation between direct costs and duration of blood transfusion with an increase in direct costs in those receiving blood transfusion over a longer duration. However, the lower direct costs in our study could be due to relatively shorter duration of blood transfusion (median age 5.7 years) and ICT (median age 2.3 years) and absence of thalassaemia-related complications due to younger age of our study population. These observations are pertinent as even though direct costs are at subsidized rates in public healthcare institutes, these are nevertheless out-of-pocket expenses. The fraction of direct costs due to travel and accommodation was just 4% as majority of our patients were residents of Mumbai, otherwise travel and accommodation of outstation patients would contribute substantially to direct costs and consequently to higher out-of-pocket expenses. Expenses on ICT and blood transfusion have been implicated for suboptimal therapy of beta-thalassaemia major in India.17 Just 11.7% of patients with beta-thalassaemia major receive optimum blood transfusion and of these, just 39% have access to ICT.19 Moirangthem and Phadke reported an even lower proportion (7.2%) of patients receiving optimum therapy, and ICT therapy was commenced later than the recommended age of 2 years.17 Few patients in India undergo haematopoietic stem cell transplant (HSCT).20 None of the previous studies had patients undergoing HSCT else inclusion of these costs would have escalated the economic burden.
In the light of the present study documenting high out-of-pocket expenditure through direct costs, several immediate and long-term measures could be implemented in India to reduce the economic burden to the family: availing government travel subsidies for outstation families, augmenting blood banks and local centres for thalassaemia daycare in various cities and towns of India so that patients are not required to travel to other states and wider implementation of thalassaemia control programmes by state governments with support from the National Health Mission to procure testing equipment and iron chelators or coordinating with support groups and nongovernmental organizations (NGOs) to secure blood transfusion and ICT free of cost.13,21 Cost for screening (₹158 100) and prenatal diagnosis (₹153 700) each accounted for 1.9% of the direct costs in our study, an insignificant proportion, indicating underutilization of these services. A long-term cost-effective strategy to reduce the burden of beta-thalassaemia major to the Government of India is prevention by carrier screening and prenatal diagnosis. A staggering cost of ₹10 000 crore (US$100 billion) estimated to treat 500 000 children with beta-thalassaemia major surviving for 50 years could be saved for every ₹1 crore (0.01 billion) spent on carrier screening of 1 lakh (0.1 million) individuals.21
Haematopoietic stem cell transplant is a one-time curative therapy for beta-thalassaemia major. Unfortunately, HSCT is underutilized in India due to cost constraints as a major cost of HSCT is out-of-pocket, whereas transfusion and chelation is free of cost or subsidized.22,23 Data from the Indian Stem Cell Registry show that just 960 patients with thalassaemia received stem cell transplant during 2012 and 2016, whereas 30% of patients (approximately 3600 affected children of 12 000 born every year) having sibling-matched donors are eligible for HSCT.13,20 As of 2017, a total of 35 centres in India have been performing HSCT for thalassaemia, including government and private hospitals.22,24,25 The cost of HSCT varies from ₹14 lakh (1.4 million) to ₹52 lakh (5.2 million) depending on the type of donor and complications.20 Several centres from the private sector have successfully managed to substantially reduce the cost of matched sibling donor HSCT for thalassaemia ranging from ₹452 821 to ₹13 00 000 by adopting practical, safe and efficacious practices.22,23,26,27 Studies from India have adequately established cost-effectiveness of one-time HSCT for thalassaemia.23,25 In this respect, government-funded schemes (Prime Minister’s relief fund) or projects such as Thalassemia Bal Sewa Yojana (a corporate social responsibility-funded project of Coal India Ltd.) offer ₹10 00 000 for allo-HSCT with matched sibling donor.23 Thus, making HSCT accessible and affordable for many more patients may curtail government’s expenditure for lifelong conventional therapy with blood transfusion and iron chelators.13,20 This requires creating widespread awareness amongst physician communities and the families of beneficiaries, especially poor patients, about government hospitals in India for availing low-cost HSCT through NGO and government support.22,24,25
Our study does have some limitations. The economic burden of beta-thalassaemia major in the study is most likely an underestimation as this was a single-centre study in a public health facility where costs are highly subsidized. Cost computations relied on parents’ recall and preservation of expenditure records. We excluded families with more than one child affected by beta-thalassaemia major. Though we did not analyse the adequacy of treatment in terms of achieving treatment goals, it is possible that the magnitude of economic burden would certainly escalate in striving for optimum management. In addition, the cross-sectional, prevalence-based approach in our study does not capture the lifetime costs, morbidity and psychological burden on the family unit of a lifelong disease such as beta-thalassaemia.
Conclusions
The economic burden of treating beta-thalassaemia major to families is considerable as a majority (94%) of the cost incurred is out-of-pocket expenses due to direct costs of ICT and blood investigations. Considering the economic impact of treating beta-thalassaemia major on individual families, healthcare system and the society, an urgent implementation of a cost-effective policy for limiting out-of-pocket expenditure, disease prevention by carrier screening and prenatal diagnosis and affordable early HSCT is required at the national level in India.
Conflicts of interest
None declared
References
- National Health Policy 2017, Ministry of Health and Family Welfare, Government of India. Available at www.nhp.gov.in/nhpfiles/national_health_policy_2017.pdf (accessed on 11 Jun 2020)
- [Google Scholar]
- Out-of-pocket expenditure and distress financing on institutional delivery in India. Int J Equity Health. 2019;18:99.
- [CrossRef] [PubMed] [Google Scholar]
- Cost-of-illness studies: Concepts, scopes, and methods. Clin Mol Hepatol. 2014;20:327-37.
- [CrossRef] [PubMed] [Google Scholar]
- Burden of genetic disorders in India. Indian J Pediatr. 2000;67:893-97.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of the national health policy of thalassaemia screening in the Islamic Republic of Iran. East Mediterr Health J. 2005;11:308-18.
- [Google Scholar]
- Psycho-social and economic impact of thalassemia major on patients' families. ISRA Med J. 2016;8:24-7.
- [Google Scholar]
- Cost-effectiveness of prenatal screening for thalassaemia in Hong Kong. Prenat Diagn. 2004;24:899-907.
- [CrossRef] [PubMed] [Google Scholar]
- Healthcare costs and outcomes of managing âthalassemia major over 50 years in the United Kingdom. Transfusion. 2016;56:1038-45.
- [CrossRef] [PubMed] [Google Scholar]
- Economic burden of beta-thalassemia/Hb E and beta-thalassemia major in Thai children. BMC Res Notes. 2010;3:29.
- [CrossRef] [PubMed] [Google Scholar]
- The economic burden of treating thalassemia in Greece. Value Health. 2014;17:A323-686.
- [CrossRef] [PubMed] [Google Scholar]
- Modified Kuppuswamy's socioeconomic scale: Social researcher should include updated income criteria. Indian J Community Med. 2013;38:185-6.
- [CrossRef] [PubMed] [Google Scholar]
- WHO guide to identifying the economic consequences of disease and injury. 2009. Geneva: WHO; Available at www.who.int/choice/publications/d_economic_impact_guide.pdf (accessed on 11 Jun 2020)
- [Google Scholar]
- Economic burden of transfusion dependent thalassemia. Indian J Pediatr. 2018;85:329-30.
- [CrossRef] [PubMed] [Google Scholar]
- Endocrine complications in thalassemias. Frequency and association with ferritin levels. Pak Ped J. 2005;29:113-19.
- [Google Scholar]
- Financial burden on the families of transfusion dependent thalassemic children. Pediatric Oncall. 2013;10:20.
- [CrossRef] [Google Scholar]
- Expenditure to treat thalassaemia: An experience at a tertiary care hospital in India. Iran J Public Health. 2010;39:78-84.
- [Google Scholar]
- Socio-demographic profile and economic burden of treatment of transfusion dependent thalassemia. Indian J Pediatr. 2018;85:102-7.
- [CrossRef] [PubMed] [Google Scholar]
- Coping with the burden of thalassemia: Aiming for a thalassemia free world. Glob J Transfus Med. 2018;3:1-5.
- [CrossRef] [Google Scholar]
- Global epidemiology of haemoglobin disorders and derived service indicators. Bull World Health Organ. 2008;86:480-7.
- [CrossRef] [PubMed] [Google Scholar]
- Access to hematopoietic stem-cell transplantation in India. J Postgrad Med. 2019;65:1-4.
- [CrossRef] [PubMed] [Google Scholar]
- National Health Mission Guidelines on Hemoglobinopathies in India 2016. Ministry of Health and Family Welfare. Government of India. Available at https://nhm.gov.in/images/pdf/programmes/RBSK/Resource_Documents/Guidelines_on_Hemoglobinopathies_in%20India.pdf (accessed on 11 Jun 2020)
- [Google Scholar]
- Pediatric hematopoietic stem cell transplantation in India: Status challenges and the way forward. Indian J Pediatr. 2017;84:36-41.
- [CrossRef] [PubMed] [Google Scholar]
- Cost effectiveness of hematopoietic stem cell transplantation compared with transfusion chelation for treatment of thalassemia major. Biol Blood Marrow Transplant. 2018;24:2119-26.
- [CrossRef] [PubMed] [Google Scholar]
- Allogenic stem cell transplantation for thalassemia major in India. Pediatr Hematol Oncol J. 2017;2:114-20.
- [CrossRef] [Google Scholar]
- Stem cell transplantation in India. Bone Marrow Transplant. 2008;42(Suppl 1):S81-4.
- [CrossRef] [PubMed] [Google Scholar]
- Low-cost matched sibling bone marrow transplant for standard-risk thalassemia in a limited-resource setting. Pediatr Hematol Oncol J. 2017;2:107-13.
- [CrossRef] [Google Scholar]
- Cost of hematopoietic stem cell transplantation in India. Mediterr J Hematol Infect Dis. 2014;6:e2014046.
- [CrossRef] [PubMed] [Google Scholar]




