Translate this page into:
Inhaled iloprost as an add-on therapy for advanced pulmonary arterial hypertension: An Indian perspective
[To cite: Bobhate P, Gupta RK, Karande T, Kulkarni S. Inhaled iloprost as an add-on therapy for advanced pulmonary arterial hypertension: An Indian perspective. Natl Med J India 2022;35:338–43.]
Abstract
Background
Pulmonary arterial hypertension (PAH) is a progressive disease with high morbidity and mortality. Risk stratification and initiation of dual or triple combination therapy has a better clinical response, especially in high-risk patients. Unfortunately, prostacyclin analogues are not marketed in India; hence, the use of these medications is limited. We report the benefits and difficulties of using iloprost inhalation in patients with advanced PAH in India.
Methods
In this prospective observational study, we included patients with group 1 PAH. Inhaled iloprost was initiated as an add-on therapy for patients who had clinical, echocardiographic or laboratory deterioration on dual oral medications. Patients with clinical instability were excluded. All patients underwent thorough clinical evaluation, detailed echocardiogram and laboratory investigations. Patients were started on inhaled iloprost 2.5 μg six times daily and closely followed up. The dose was escalated if necessary. On follow-up, clinical echocardiographic and laboratory evaluation was done on all patients.
Results
Fourteen patients (11 women) with a median age of 32 years (2–66 years) with group 1 PAH were started on inhaled iloprost as an add-on therapy. Improvement in clinical parameters, WHO functional class, echocardiographic-derived right ventricular function, and N-terminal pro-brain natriuretic peptide (NT-pro-BNP) levels were observed in 10 of 14 patients. A median increase of 31% (4.2, 106%) in the distance travelled during 6-minute walk test, a median increase of 45% (–20, 120%) in right ventricular fractional area change, a median increase of 27% (–16.7, 60%) in tricuspid annular peak systolic excursion and a median decrease of 36.7% (–69.6, 17.2%) in NT-pro-BNP levels were observed after initiation of medication. Three patients had progression of symptoms and were then referred for lung/heart–lung transplant. One patient developed progression of symptoms after an excellent initial response and transitioned to subcutaneous treprostinil. Improvement in clinical, echocardiographic and laboratory features allowed us to successfully perform surgical Potts shunt in 2 patients. The medications were well tolerated with minimal and transient side-effects. There were no deaths.
Conclusion
Inhaled iloprost can be used with acceptable benefits and minimal side-effects in patients with PAH.
INTRODUCTION
Pulmonary arterial hypertension (PAH) is a progressive disease with high morbidity and mortality. The decrease in prostacyclin expression and excretion of prostacyclin metabolites provided a rationale for treating patients of PAH with prostacyclin analogues.1,2 Prostacyclin analogues have potent anti-inflammatory, vasodilator and antithrombotic effects in PAH.3 Intravenous epoprostenol was the first drug approved for PAH use after a randomized controlled trial demonstrated improvement in exercise capacity, cardiopulmonary haemo-dynamics and survival compared to conventional therapy.4 Since the introduction of epoprostenol, prostanoids with better stability and easier route of administration have shown morbidity and mortality benefits in PAH patients.5,6 Iloprost is a short-acting, stable prostacyclin analogue administered through inhalation, thus avoiding the systemic side-effects of parental prostanoids. Inhalation of iloprost dilates the well-ventilated areas of the pulmonary artery, thus ensuring optimal gas exchange and preventing ventilation–perfusion mismatch. The safety and efficacy of iloprost in managing patients with PAH with advanced functional class have been documented.7,8
None of the prostacyclin analogues are currently marketed in India. Although they can be imported for personal use, the cost is prohibitory. This limits the use of these life-saving medications, especially in advanced pulmonary hypertension. We describe the benefits and difficulties of using iloprost in patients with PAH of advanced functional class from a tertiary care centre in India.
METHODS
We did this prospective observational study of patients with PAH. PAH was defined as mean pulmonary artery pressure of >20 mmHg with pulmonary artery wedge pressure of <15 mmHg and pulmonary vascular resistance indexed to body surface area of >3 Wood units m2. All patients underwent thorough clinical evaluation, detailed echocardiogram and laboratory investigations. Patients with clinical instability were not considered for inclusion.
A 6-minute walk test was performed in 11 of 14 patients before initiation of therapy. Two patients in whom the 6-minute walk test was not performed were <6 years of age. Clinical evaluation, echocardiogram, N-terminal pro-brain natriuretic peptide (NT-pro-BNP) and 6-minute walk test were repeated at 3 and 6 months post-initiation of iloprost. All the other medications which the patient was taking before initiation of iloprost were continued during the follow-up. We defined clinical improvement as an increase in 6-minute walk test of >25% or a reduction in NT-pro-BNP of >25%. Progression of the disease was defined as listing for lung/heart–lung transplant or death.
Pharmacotherapy with inhaled iloprost
Patients presenting to the pulmonary hypertension clinic who had deterioration of functional class on maximal medical therapy available in the country were counselled regarding various prostacyclin analogues. Iloprost was selected due to ease of administration, effectiveness, availability and cost.9 In addition, the inhaled route offers a degree of pulmonary selectivity, avoiding potentially harmful systemic hypotension observed with intravenous prostanoids.
Inhaled iloprost was started under medical supervision in a dose of 2.5 μg six times daily. Vital parameters, including saturation, heart rate and blood pressure, were monitored before and up to 2 hours post-initiation of iloprost in daycare. Patients were then discharged home to continue the medications every 4 hours and report if they develop any side-effects. Patients were followed up at regular intervals in the pulmonary hypertension clinic as per protocol. The day of initiation of iloprost was labelled as time #0 and the day of last follow-up was labelled as time #1. All the medications the patient was taking before initiation of iloprost were continued during the follow-up.
Institutional ethics committee approval was obtained as a part of the PAH registry.
Statistical analysis
The statistical analysis was based on clinical, laboratory and echocardiographic datasets and was done using SPSS 20 software. The Wilcoxon’s signed-rank test was used for pairwise comparison of data at baseline and at follow-up.
RESULTS
The median age of the patients was 32 years (2–66 years). There were 11 women. Six patients had idiopathic PAH, 3 had PAH out of proportion to the congenital heart disease (CHD) and 5 patients had residual PAH after repair of CHD (3 after ventricular septal defect closure, 1 after atrial septal defect closure, and one after arterial switch for transposition of great arteries in the neonatal period). All the patients were on phosphodiesterase-5 inhibitors and endothelin receptor antagonist for at least 3 months before the study. Patients who had deterioration in functional class, echocardiographic or laboratory parameters on dual therapy were counselled for starting iloprost. Five of 14 patients had right heart failure (ascites and oedema feet), whereas 6 of 14 had a history of syncope on minimal exertion (Table I). Eight of 14 patients were in WHO functional class (WHO FC) IV, and the remaining were in WHO FC III. The median saturation (SpO2) in the right upper limb at rest was 88% (70%–98%). Seven of 14 patients, who had saturations <92% at baseline, were on continuous, and 2 of 14 were on nocturnal oxygen therapy. The median distance covered during the 6-minute walk test was 119 metres (90–190 m). The mean (SD) delay between starting iloprost and acquiring the medication was 15 (3) days.
| Age (years)/Sex | Diagnosis | Features of RHF | Syncope | SpO2 | at rest | Requirement of O2 | Follow-up (months) |
|---|---|---|---|---|---|---|---|
| 2/M | TGA, S/p ASO | No | Yes | 9 0 | No | 20.3 | |
| 52/M | IPAH | Yes | Yes | 8 8 | Yes | 18.2 | |
| 20/F | S/p VSD closure | No | Yes | 9 8 | No | 8.4 | |
| 13/F | IPAH | No | Yes | 9 8 | No | 20.2 | |
| 47/F | PAH out of prop. CHD | No | No | 8 8 | Yes | 13.2 | |
| 50/F | S/p ASD closure | Yes | No | 9 2 | Nocturnal | 20.6 | |
| 42/F | PAH out of prop. CHD | No | No | 9 2 | Nocturnal | 11.6 | |
| 66/F | IPAH | Yes | No | 8 7 | Yes | 12.6 | |
| 32/F | IPAH | No | Yes | 7 9 | Yes | 37.0 | |
| 17/F | IPAH | No | Yes | 8 6 | Yes | 9.3 | |
| 5/F | IPAH | Yes | No | 7 6 | Yes | 9.3 | |
| 36/F | PAH out of prop. CHD | Yes | No | 9 0 | Yes | 9.4 | |
| 16/M | Post-VSD closure | No | No | 9 8 | No | 6.1 | |
| 33/F | Post-VSD closure | Yes | No | 9 2 | No | 13.1 | |
RHF right heart failure SpO2 oxygen saturation TGA S/p ASO transposition of great arteries status post-arterial switch operation IPAH idiopathic pulmonary arterial hypertensionv PAH out of prop. CHD pulmonary arterial hypertension out of proportion to congenital heart disease ASD atrial septal defect VSD ventricular septal defect
Echocardiographic and laboratory features
As measured by tricuspid annular plane systolic excursion (TAPSE) and right ventricular fractional area change (RVFAC), right ventricular longitudinal function was depressed in all patients (Table III). The median TAPSE was 12 (9–17) mm, and the median RVFAC was 18 (8–26) mmHg. The median left ventricular eccentricity index was 3.85 (2.8–4.3), and the heart rate-corrected pulmonary artery acceleration time (PAAT) was 64.5 (47–79). The median NT-pro-BNP was 1717 (468– 27 893) pg/ml.
| Serial number | NT-pro-BNP (time #0) | NT-pro-BNP (time #1) | Percentage change (NT-pro- BNP) | 6MWTD (time #0) | 6MWTD (time #1) | Percentage change (6MWTD) |
|---|---|---|---|---|---|---|
| 1 | 27 893 | 9983 | –64.2 | – | – | – |
| 2 | 1655 | 1940 | 17.2 | 9 6 | 100 | 4.2 |
| 3 | 5717 | 6184 | 8.2 | 110 | 115 | 4.5 |
| 4 | 1238 | 664 | –46.4 | 190 | 225 | 18.4 |
| 5 | 756 | 234 | –69.0 | 138 | 178 | 29.0 |
| 6 | 468 | 146 | –68.8 | 165 | 220 | 33.3 |
| 7 | 1128 | 662 | –41.3 | 9 8 | 136 | 38.8 |
| 8 | 3345 | 3790 | 13.3 | 110 | 118 | 7.3 |
| 9 | 7865 | 7800 | –0.8 | 9 0 | 104 | 15.6 |
| 10 | 1268 | 996 | –21.5 | 9 6 | 198 | 106.3 |
| 11 | 11 435 | 5647 | –50.6 | – | – | – |
| 12 | 1780 | 542 | –69.6 | 140 | 225 | 60.7 |
| 13 | 986 | 665 | –32.6 | 128 | 187 | 46.1 |
| 14 | 2568 | 1986 | –22.7 | 134 | 198 | 47.8 |
| Median (25, | 1717 (1092, 6254) | 1468 (632, 5781) | –36.7 | 119 (96, 139) | 182.5 (116, 214) | +31.2 |
| 75 percentile) | ||||||
| p value | 0.03 | 0.04 |
| Serial number | RVFAC (time #0) | RVFAC (time #1) | Percentage change (RVFAC) | TAPSE (time #0) | TAPSE (time #1) | Percentage change (TAPSE) |
|---|---|---|---|---|---|---|
| 1 | 1 0 | 2 2 | 120 | 1 1 | 1 4 | 27.3 |
| 2 | 8 | 9 | 1 3 | 1 2 | 1 0 | –16.7 |
| 3 | 1 5 | 1 6 | 7 | 1 4 | 1 2 | –14.3 |
| 4 | 1 8 | 3 0 | 6 7 | 1 7 | 2 2 | 29.4 |
| 5 | 2 2 | 3 2 | 4 5 | 1 3 | 1 5 | 15.4 |
| 6 | 1 8 | 3 4 | 8 9 | 1 6 | 1 9 | 18.8 |
| 7 | 2 2 | 3 2 | 4 5 | 1 5 | 1 9 | 26.7 |
| 8 | 2 0 | 1 8 | –10 | 1 2 | 1 0 | –16.7 |
| 9 | 2 5 | 2 0 | –20 | 1 1 | 1 0 | –9.1 |
| 1 0 | 2 6 | 3 8 | 4 6 | 1 4 | 2 0 | 42.9 |
| 1 1 | 2 0 | 2 4 | 2 0 | 1 0 | 1 6 | 60.0 |
| 1 2 | 1 4 | 2 8 | 100 | 1 2 | 1 8 | 50.0 |
| 1 3 | 1 0 | 1 5 | 5 0 | 9 | 1 4 | 55.6 |
| 1 4 | 1 4 | 2 0 | 4 3 | 1 2 | 1 7 | 41.7 |
| Group median (25, 75 percentile) | 18 (13, 22) | 23 (18, 32) | 4 5 | 12 (11, 15) | 15.5 (11, 19) | +27 |
| p value | 0.006 | 0.01 |
RVFAC right ventricular fractional area change TAPSE tricuspid annular peak systolic excursion
Individual drug response to inhaled iloprost
The patients were followed up for a median of 13.2 (8.4–37) months. There were no deaths during iloprost therapy. Three patients (patients number 2, 3 and 8) were ultimately listed for heart and lung transplant due to disease progression. Ten patients showed improved functional class, distance travelled during a 6-minute walk test, echocardiographic parameters and laboratory investigations (Table II). After initial improvement, 2 patients underwent surgical Potts shunt. Both of these patients are alive and well. The creation of Potts shunt allowed us to stop the iloprost inhalation in these patients 6 months after surgery. After a favourable initial response, at 30 months post-initiation of iloprost, one patient had progression of symptoms and right heart failure. She required an increase in the dose of iloprost to 5 μg nine times daily. Even after increasing the iloprost dose, lack of clinical and biochemical improvement subsequently warranted a change to subcutaneous treprostinil (Fig. 1).
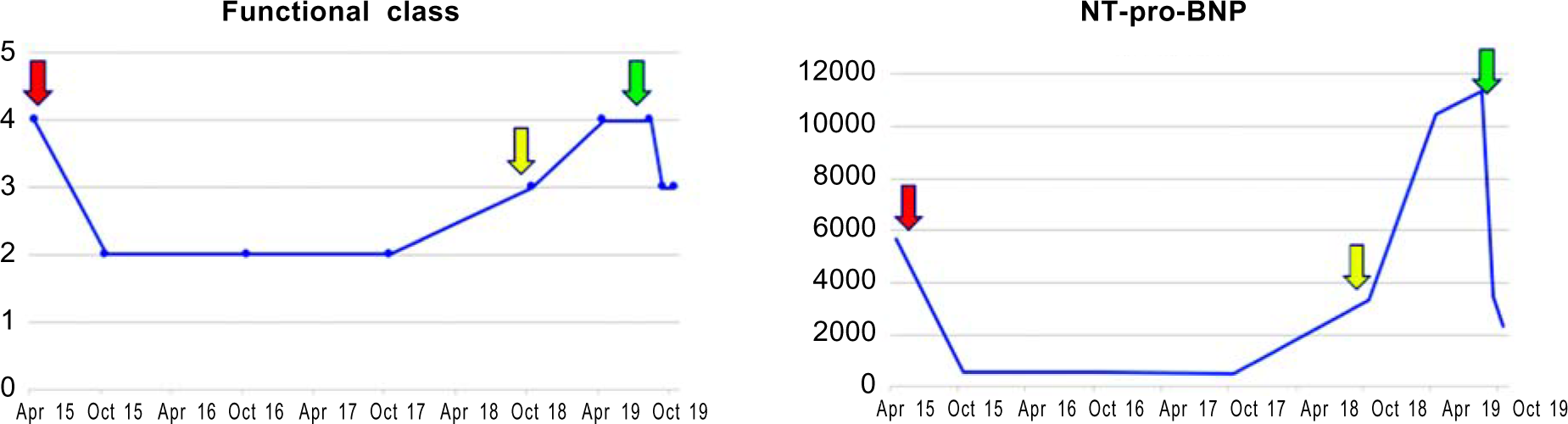
- Clinical and laboratory changes in a patient who presented to us in functional class IV. Inhaled iloprost was started at the dose of 2.5 μg 6 times daily (red arrow). She had an excellent response to inhaled iloprost which was sustained for 2 years. Gradual deterioration of clinical status was then noted. The dose of iloprost was increased (yellow arrow) without much improvement. Inhaled iloprost was then replaced with subcutaneous treprostinil (green arrow) with good clinical response
Improvement in clinical parameters and 6-minute walk test
Ten of 14 patients demonstrated improvement in WHO FC of at least one grade after starting inhaled iloprost (Table IV). Improvement in features of right heart failure was observed in 4 of 5 patients, and 4 of 6 patients who had syncope at the time of initiation of iloprost were free of the symptoms at follow-up. Improvement in baseline oxygen saturation, allowing a decrease in oxygen therapy, was observed in 6 of 9 patients. A median increase of 31% (4.2%–106%) in the distance travelled during the 6-minute walk test was observed (Fig. 2).
| Change in clinical status | WHO functional class at the initiation of iloprost* | Total | |
|---|---|---|---|
| III | IV | ||
| Clinical improvement | 6 | 4 | 1 0 |
| No clinical improvement | 0 | 4 | 4 |

- The percentage change in the 6-minute walk test (a) and N-terminal pro-brain natriuretic peptide (b) from initiation of iloprost (time #0) and at last follow-up (time #1)
Improvement in echocardiographic parameters and N-terminal pro-brain natriuretic peptide
The addition of inhaled iloprost improved echocardiographic variables of right ventricular function and NT-pro-BNP at follow-up. TAPSE improved from a median of 12 (9–17) mm to 15 (10–22) mm (p=0.02). RVFAC improved from a median of 18% (8%–26%) to 23% (9%–38%; p=0.03). Similar improvements were observed in the left ventricular eccentricity index and pulmonary acceleration time (Table II). Figure 3 shows the change in patients’ echocardiographic parameters between time # 0 and time # 1.

- The percentage change in the echocardiographic parameters from initiation of iloprost (time #0) and at last follow-up (time #1): (a) left ventricular eccentricity index; (b) right ventricular fractional area change; (c) heart rate corrected pulmonary artery acceleration time; and (d) tricuspid annular peak systolic excursion
NT-pro-BNP decreased from median of 4459 (1655–27 893) pg/mol to 996 (146–9983; p=0.03; Fig. 2).
Adverse events of inhaled iloprost
There were no deaths during the use of iloprost. Minor side-effects included nasal stuffiness (8/14, 54%), headache (3/14, 18%), nausea (1/14, 7.5%) and jaw pain (1/14, 7.5%). Most of the side-effects were self-limiting, and none of the patients stopped iloprost inhalation because of the adverse events.
DISCUSSION
Progressive PAH is characterized by pulmonary vascular remodelling, leading to elevated pulmonary vascular resistance, right ventricular failure and death.10,11 PAH-associated mortality has decreased over the past 2 decades, mostly secondary to increased awareness of the disease and its multiple aetiologies, more accurate diagnosis, better risk stratification and upfront dual or triple combination therapies.12 Prostacyclin analogues form a cornerstone for the management of PAH. Those patients who remain in the intermediate- or high-risk category after 3–6 months of oral combination therapy or deteriorate to the high-risk group on treatment need to be started on prostacyclin analogues or referred for lung–heart and lung transplant.12 However, prostacyclin analogues are currently not marketed in India and have to be imported for personal use, limiting their widespread use. Lung or heart–lung transplant is cost-prohibitive with limited availability and guarded long-term outcomes.13
Improvement in clinical, echocardiographic and laboratory features
An improvement in clinical features, distance travelled during a 6-minute walk test, echocardiographic parameters and NTpro-BNP were observed after initiating the patients on inhaled iloprost. Most of the above factors have been shown to predict clinical outcomes in adults and children with PAH.12 Hence, improvement in these factors could translate into better clinical outcomes. However, the disease progressed in 3 patients resulting in listing for heart and lung transplant. The benefits of the use of prostacyclin analogues have been well documented in the literature. The use of prostacyclin early in the disease process is associated with better long-term outcomes.14 In our study, good clinical response was observed in 100% of the patients in functional class III compared to 50% in patients with class IV symptoms.
Bridge to Potts shunt/lung transplant
Once started, prostacyclin analogues have to be continued for life or until the patient has a procedure such as a lung or heart– lung transplant. The same might be challenging in the Indian scenario. The creation of an unrestrictive connection between the left pulmonary artery and the descending aorta has been shown to offer symptomatic improvement and decrease the need for prostacyclin analogues in patients with advanced PAH.15 However, it is a high-risk procedure, and the patients with preserved right ventricular function tolerate the procedure better.16 In our study, 2 patients underwent successful creation of Potts shunt three months post-initiation of iloprost therapy. Further improvement of functional class and right ventricular function post-surgery allowed us to stop iloprost three months after surgery.
Long-term effects
The beneficial effects of prostacyclin analogues might wane over time. This is believed to be secondary to the disease progression or could be secondary to tolerance to prostanoid therapy.17 In our series, 1 patient required conversion to subcutaneous treprostinil with an excellent clinical response. This could be secondary to the difference in prostacyclin receptor activation by iloprost and treprostinil.18
Adverse events and limitations of use
Adverse events reported with inhaled iloprost are usually mild, ranging from headache, flushing, jaw pain and nasal stuffiness. Most of our patients had minor side-effects, which were transient and tolerated well. None of the patients stopped medications due to the side-effects.
Challenges to use of prostacyclin analogues in India
The limitations of using prostacyclin analogues in India are its availability, the time required to procure the medication and its cost. The average delay from the date of prescription to the drug acquisition in our experience was 15 (3) days. Hence, it cannot be used as rescue therapy and needs to be started at the first sign of clinical, echocardiographic and laboratory deterioration. The cost of the drug is another limitation. Over the past 3 years, with consistent use, the cost of administering iloprost has decreased from approximately `150 000 to `20 000/month. However, it is still twice the per capita monthly income in India. The financial implications might prevent the patients from continuing the medications for a longer time. To circumvent this, we suggest using iloprost as a bridge to Potts shunt or heart–lung transplant.
Iloprost is a relatively short-acting drug and has to be taken 6–9 times daily, with each nebulization lasting for approximately 15 minutes. Need to take this medication frequently leaves limited time for sleep and rest. We circumvented this problem by giving a drug holiday in the night and dividing the remaining doses equally throughout the day.
Our study is limited by a small sample size and short duration of follow-up.
Conclusion
Our study shows that inhaled iloprost can be used with acceptable benefit and minimal side-effects in Indian patients. We have also shown its use as a bridge to Potts shunt to mitigate the risks of a high-risk procedure.
Conflicts of interest
None declared
References
- Prostacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertension. Am J Respir Crit Care Med. 1999;159:1925-32.
- [CrossRef] [PubMed] [Google Scholar]
- An imbalance between the excretion of thromboxane and prostacyclin metabolites in pulmonary hypertension. N Engl J Med. 1992;327:70-5.
- [CrossRef] [PubMed] [Google Scholar]
- Prostacyclin therapies for the treatment of pulmonary arterial hypertension. Eur Respir J. 2008;31:891-901.
- [CrossRef] [PubMed] [Google Scholar]
- A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med. 1996;334:296-301.
- [CrossRef] [PubMed] [Google Scholar]
- Continuous subcutaneous infusion of treprostinil, a prostacyclin analog, in patients with pulmonary arterial hypertension: A double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2002;165:800-4.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of beraprost sodium, an oral prostacyclin analog, in patients with pulmonary arterial hypertension: A randomized, double-blind, placebo-controlled trial. J Am Coll Cardiol. 2002;39:1496-502.
- [CrossRef] [PubMed] [Google Scholar]
- Inhaled iloprost for severe pulmonary hypertension. N Engl J Med. 2002;347:322-9.
- [CrossRef] [PubMed] [Google Scholar]
- Combining inhaled iloprost with bosentan in patients with idiopathic pulmonary arterial hypertension. Eur Respir J. 2006;28:691-4.
- [CrossRef] [PubMed] [Google Scholar]
- Cost effectiveness of prostacyclins in pulmonary arterial hypertension. Appl Health Econ Health Policy. 2012;10:175-88.
- [CrossRef] [PubMed] [Google Scholar]
- Pathology and pathobiology of pulmonary hypertension: State of the art and research perspectives. Eur Respir J. 2019;53:1801887.
- [CrossRef] [PubMed] [Google Scholar]
- Pathophysiology of the right ventricle and of the pulmonary circulation in pulmonary hypertension: An update. Eur Respir J. 2019;53:1801900.
- [CrossRef] [PubMed] [Google Scholar]
- Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J. 2019;53:1801889.
- [CrossRef] [PubMed] [Google Scholar]
- Lung transplantation in children with idiopathic pulmonary arterial hypertension: An 18-year experience. J Heart Lung Transplant. 2011;30:1148-52.
- [CrossRef] [PubMed] [Google Scholar]
- Prostacyclin in primary pulmonary hypertension. Eur Heart J. 1996;17:18-24.
- [CrossRef] [PubMed] [Google Scholar]
- Palliative Potts shunt for the treatment of children with drug-refractory pulmonary arterial hypertension: Updated data from the first 24 patients. Eur J Cardiothorac Surg. 2015;47:e105-10.
- [CrossRef] [PubMed] [Google Scholar]
- Potts shunt improves right ventricular function and coupling with pulmonary circulation in children with suprasystemic pulmonary arterial hypertension. Circ Cardiovasc Imaging. 2018;11:e007964.
- [CrossRef] [PubMed] [Google Scholar]
- Children with pulmonary arterial hypertension and prostanoid therapy: Long-term hemodynamics. J Heart Lung Transplant. 2013;32:546-52.
- [CrossRef] [PubMed] [Google Scholar]
- Binding and activity of the prostacyclin receptor (IP) agonists, treprostinil and iloprost, at human prostanoid receptors: Treprostinil is a potent DP1 and EP2 agonist. Biochem Pharmacol. 2012;84:68-75.
- [CrossRef] [PubMed] [Google Scholar]




