Translate this page into:
Intravenous ferric carboxymaltose rapidly increases haemoglobin and serum ferritin among pregnant females with moderate-to-severe anaemia: A single-arm, open-label trial
Corresponding Author:
Partha Haldar
Department of Community Medicine, Centre for Community Medicine, All India Institute of Medical Sciences, New Delhi
India
parthahaldar@outlook.com
| How to cite this article: Kant S, Haldar P, Malhotra S, Kaur R, Rath R, Jacob OM. Intravenous ferric carboxymaltose rapidly increases haemoglobin and serum ferritin among pregnant females with moderate-to-severe anaemia: A single-arm, open-label trial. Natl Med J India 2020;33:324-328 |
Abstract
Background. Infusion of ferric carboxymaltose (FCM) can correct iron deficiency anaemia in the second or third trimester of pregnancy. However, large-scale public health use of FCM is constrained by two issues. First, there is limited evidence on the efficacy and safety profile of FCM in the Indian context. Second, logistic challenges in administering FCM at a subdistrict healthcare setting have not been assessed. We aimed to measure the mean increase in haemoglobin (Hb) level 2 weeks after infusion of FCM to pregnant females with moderate-to-severe anaemia attending a subdistrict hospital in India.Methods. During June–December 2016, we did a single-arm, open-label trial among pregnant females with a gestation of 16–32 weeks, Hb 5.0–9.9 g/dl. FCM was infused (per Ganzoni formula) in a single session up to a maximum of 1000 mg of iron. Hb and s-ferritin levels were measured at recruitment, 2 weeks post-infusion and at delivery. Adverse events were noted.
Results. Seventy-seven pregnant females were enrolled with mean (SD) age 23.2 (3.1) years, gestation 27.6 (3.8) weeks and Hb 8.4 (0.9) g/dl. At 2 weeks post-infusion (n=63), the mean Hb level increased by 1.9 g/dl (95% confidence interval [CI] 1.6–2.3) and at delivery (n = 64) by 2.9 g/dl (95% CI 2.4–3.4). The median (interquartile range) (in ng/ml) for serum ferritin at baseline (n = 68), 2 weeks post-FCM infusion (n = 61) and at delivery (n = 39) was 6.3 (5.1–11.7), 275.4 (186.4–330.3) and 61.3 (42.5–132.0), respectively. No major adverse effects were reported.
Conclusion. Infusion of FCM rapidly corrected anaemia, sustained its effect till delivery and replenished body iron reserves. FCM is safe and effective in treating anaemia in pregnant females in the second and third trimester at the subdistrict healthcare setting in India.
Introduction
More than half the pregnant females (58%) in India suffer from anaemia, defined as a haemoglobin (Hb) level of <11 g/dl, at any time during pregnancy.[1] The global reported range of prevalence of anaemia during pregnancy is 35%–75% in developing countries.[2] The primary cause of anaemia during pregnancy is iron deficiency.[3] Anaemia in pregnancy could result in adverse health consequences both for the mother and the child.[2]
Oral iron supplementation is the first-line treatment for iron deficiency anaemia. Parenteral iron is preferred when oral iron is poorly tolerated affecting compliance, or when rapid restoration of Hb and iron store is required. Poor adherence to oral iron therapy among pregnant females in India has been extensively documented.[1] Infusion of ferric carboxymaltose (FCM) can correct iron deficiency anaemia in the second or third trimester of pregnancy. However, large-scale public health use of FCM in India is constrained by two issues. First, there is limited evidence on its efficacy and safety profile in the Indian context. Second, logistic challenges in administering FCM at a subdistrict healthcare setting have not been assessed.[4] We measured the mean increase in Hb level 2 weeks after infusion of FCM to pregnant females with moderate-to-severe anaemia attending a subdistrict hospital (SDH). Our secondary objectives were to measure the median increase in serum ferritin level 2 weeks after infusion of FCM and to measure Hb and serum ferritin levels at the time of delivery.
Methods
We did a single-arm, open-label trial which included females in the second or third trimester of pregnancy with moderate-to-severe anaemia (Hb 5.0–9.9 g/dl) who attended the antenatal care (ANC) clinic of a SDH.
Study setting
The SDH was situated at Ballabgarh block of Faridabad district in the state of Haryana in northern India. The hospital provided ANC thrice a week. The study period was June 2016 to December 2016.
Inclusion criteria
We included pregnant females with a gestation period of 16–32 weeks, Hb 5.0–9.9 g/dl, and evidence of iron deficiency anaemia (microcytic hypochromic anaemia on peripheral blood smear); and those who gave written informed consent and agreed to provide blood samples at the three time-points.
Exclusion criteria
We excluded those who had renal or hepatic impairment, whose Hb was <5.0 g/dl; those who had a history of parenteral iron administration/blood transfusion during the current pregnancy; those allergic to iron preparations; or, known cases of thalassaemia, sickle cell anaemia or haemolytic anaemia.
Sample size
Using the formula shown below, where, Z1-α (5% level of significance, two-tailed) is 1.96; Z1-β (90% power) 1.28; difference in Hb 1 (SD 2.3) g/dl; a minimum of 56 pregnant females were required, which was adjusted for a 35% loss to follow-up, and rounded off to 90. The assumed SD was based on a study by Patel et al.[5] The loss to follow-up was an estimate that reflected the retention of females registered for ANC at the hospital till their delivery.
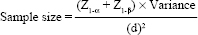
Study tools
A self-designed questionnaire was used to record the socio-demographic details. Hb was measured using the Hemocue method (HemoCue AB; Angelholm, Sweden); chemiluminescent assay (Access-2 Beckmann-coulter) was used to estimate serum ferritin. Peripheral blood smear was stained manually with Leishman stain and examined under the microscope.
Infusion of ferric carboxymaltose
The total iron requirement was calculated using the Ganzoni formula (2.4×pre-pregnancy weight×[target Hb–current Hb level]+iron store). Target Hb level was taken as 12 g/dl and allowance for iron store was 500 mg. The calculated dose of iron was rounded off to the nearest 50 mg. The dose was capped to a maximum of 1000 mg, diluted in 100 ml of normal saline and infused over 10–15 minutes. Participants were observed for any adverse event for 30 minutes following infusion of FCM.
Data collection
We collected a blood sample from each participant to measure the levels of Hb and serum ferritin before infusion of FCM (baseline). Hb and serum ferritin levels were measured at 2 weeks after infusion of FCM (first follow-up) and also at the time of delivery (second follow-up). Adverse events were observed for 30 minutes following infusion of FCM (early events) and also elicited 2 weeks after the infusion (late events).
Outcome measures
The mean increase in Hb level at 2 weeks after the infusion of FCM was the primary outcome. The secondary outcome measures were median increase in serum ferritin level 2 weeks after the infusion of FCM and Hb and serum ferritin levels at the time of delivery.
Statistical analysis
Data were entered into EpiInfo version 6 and analysed in Stata version 13.0 (Stata Statistical Software: Release 13. College Station, TX, USA: StataCorp LP).[6] Results were expressed as frequencies (%), mean (SD)/median (interquartile range [IQR]). Statistical tests used were paired t-test (parametric) and Wilcoxon signed-rank test (non-parametric). A value of p<0.05 was considered statistically significant. We analysed the change in Hb levels between different time-points by simple cross-tabulations.
Ethics
The study followed Good Clinical Practices as prescribed by the Indian Council of Medical Research. The study was approved by the Ethics Committee of the All India Institute of Medical Sciences, New Delhi and was registered at the Clinical Trial Registry India (registration no.: 2016/01/010594). All personal identifiers were delinked from the main dataset to ensure confidentiality.
Results
A total of 112 females were screened for eligibility, of which 17 were excluded, resulting in 95 who were eligible and consented to participate in the study [Figure - 1]. Eligible consenting pregnant females were advised to come on the following day for infusion of FCM. Fifteen of the 95 did not turn up on the day of the appointment; hence FCM could be started on 80 females. The infusion of FCM was stopped before the complete dose could be given in 3—1 complained of abdominal pain and the other 2 of dizziness and palpitation. All 3 were kept under observation for 1–2 hours before discharging them. Thus, a total of 77 pregnant females received an infusion of FCM.
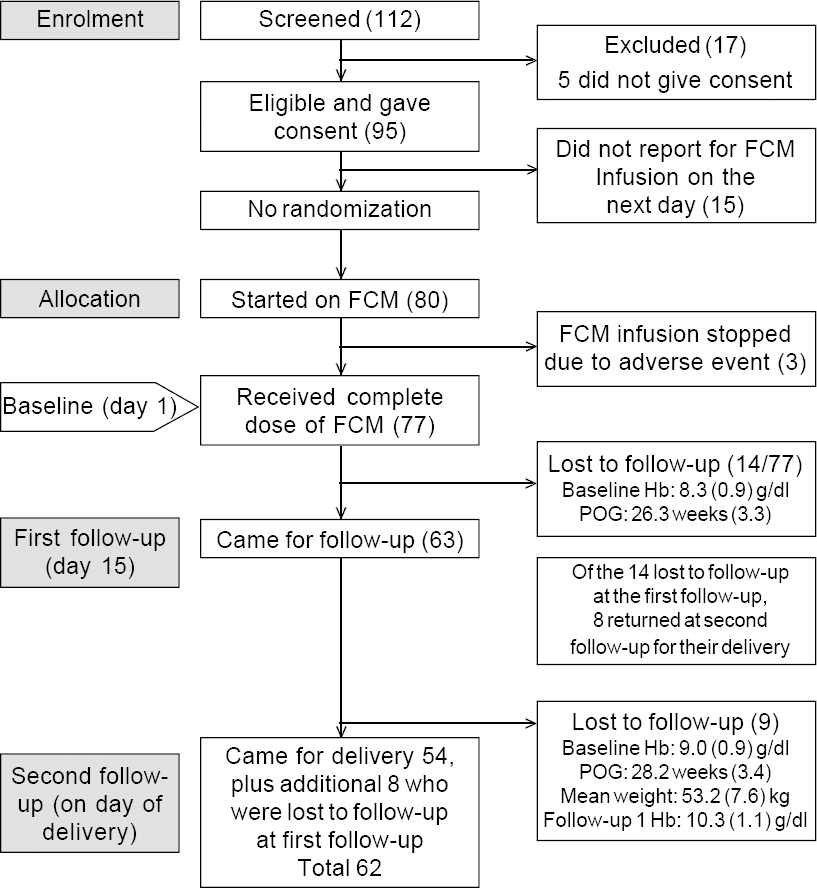 |
| Figure 1: Flowchart for the study POG period of gestation |
Description of characteristics at the three time-points
At baseline, the mean (SD) age was 23.2 (3.1) years, mean (SD) gestation period was 27.6 (3.8) weeks and mean (SD) Hb level 8.4 (0.9) g/dl. The mean weight was 53.2 (7.5) kg and the mean dose of FCM 862.5 (161) mg. Between the baseline and first follow-up, 14 females were lost to follow-up [Figure - 1]. For the remaining 63 females, who completed the first follow-up, the mean (SD) Hb level was 10.3 (1.1) g/dl.
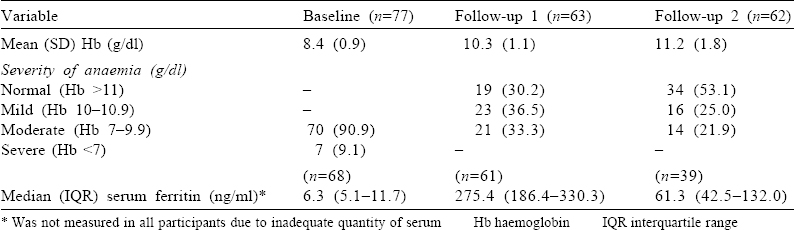
Eight of the 14 participants who were lost at the first follow-up returned at the time of their delivery (second follow-up). Between the first and second follow-up, an additional 9 participants were lost to follow-up. Thus, a total of 62 females were available for the second follow-up [Figure - 1]. At the second follow-up, the mean (SD) Hb level was 11.2 (1.8) g/dl. The median (IQR) serum ferritin level increased from 6.3 (5.1–11.7) ng/ml at baseline to 275.4 (186.4–330.3) ng/ml at the first follow-up [Table - 1]. The serum ferritin level could not be measured for all due to inadequate quantity of serum.
Change in mean Hb and median serum ferritin levels
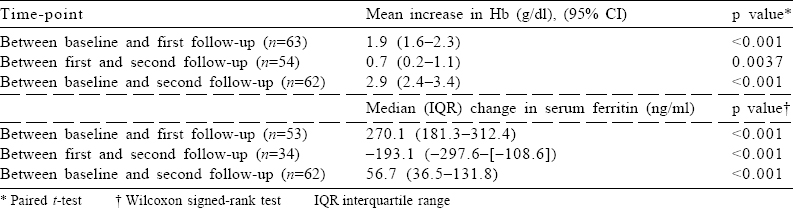
Between baseline and first follow-up, the mean Hb level increased by 1.9 g/dl (95% CI 1.6–2.3), while between baseline and second follow-up, it increased by 2.9 g/dl (95% CI 2.4–3.4; [Table - 2]). The median (IQR) (in ng/ml) for serum ferritin at baseline (n=68), 2 weeks post-infusion of FCM (n=61) and at delivery (n=39) was 6.3 (5.1–11.7), 275.4 (186.4–330.3) and 61.3 (42.5–132.0), respectively [Table - 1].
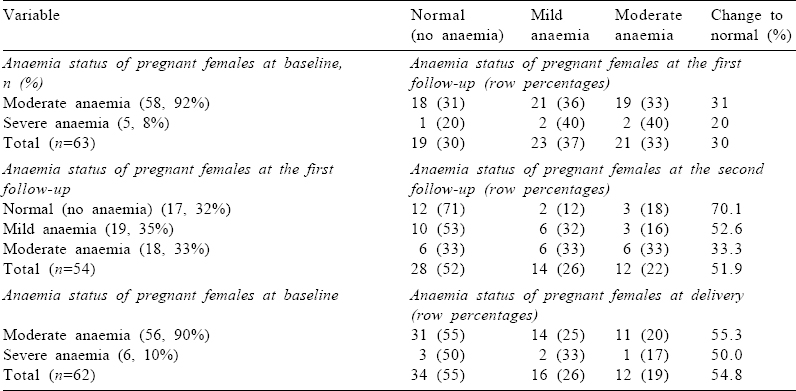
Change in proportion of anaemic females
At baseline, the proportion of pregnant females with moderate and severe anaemia was 92% and 8%, respectively [Table - 3]. At the first follow-up (at day 15), no pregnant female had severe anaemia. Almost one-third (30%) of females achieved the normal Hb level (Hb >11 g/dl) at the first follow-up. This proportion increased over time, and more than half (55%) the anaemic females achieved the normal Hb level at the time of delivery, i.e. at the second follow-up [Table - 3].
Adverse events
Of the 77 participants who received FCM, 19 (24.7%) reported any adverse event. The three most frequent adverse events reported were generalized body ache, fever and pain abdomen. None of the events were serious in nature, and all were managed symptomatically.
Discussion
We report here the effect of infusion of FCM on mean Hb level among pregnant females with moderate-to-severe anaemia, who sought ANC at an SDH in a northern Indian town. Two weeks following the infusion of FCM, the mean Hb level increased by 1.9 g/dl. The rising trend in Hb level was sustained, and at the time of delivery, the mean increase in Hb level was 2.9 g/dl. Consequently, by the time of delivery, none of the pregnant females had severe anaemia; and 34 of 62 (55%) became non-anaemic. Not only was the status of anaemia corrected but also the iron reserves of the body, as measured by median serum ferritin level, were replenished.
We found seven Indian studies that assessed the effect of infusion of FCM in females.[5],[7],[8],[9],[10],[11],[12] The reported mean increase in Hb level in these studies was in the range 2.4–5.5 g/dl. The change in serum ferritin level, reported by only five of them, was in the range 30–307 ng/ml.[5],[7],[8],[9],[10],[11],[12] The differences in the study population, eligibility criteria, calculation of iron requirement and study design do not permit us to directly compare our findings with those of earlier studies.
A recent meta-analysis reported that following infusion of FCM, the mean Hb level increased steadily over weeks 1–6, with a mean increase of about 3 g/dl by week 6 and sustained at that level to week 12.[13] The second follow-up in our study was on average 12 weeks after the infusion of FCM, and the mean increase in Hb level was 2.9 g/dl. Thus, the result of our study is aligned to the conclusion drawn in the meta-analysis.
One indication for the use of FCM is a rapid correction of anaemia among pregnant females. Therefore, we decided to measure the Hb level 2 weeks after infusion of FCM. We observed that all 5 severely anaemic, and 39 of 58 moderately anaemic participants either became non-anaemic or improved to a less severe category of anaemia. Thus, we were able to show that infusion of FCM does indeed result in a rapid increase in the Hb level. However, in the literature, there is no uniformity about the time interval when the first follow-up measurement of Hb level is done. It has been reported that Hb level may keep on increasing up to 6 weeks after infusion of FCM. Thus, the proportion of those whose anaemia may get corrected could also be a function of time, at least up to 6 weeks. For easy comparability of the effectiveness of FCM across studies, it is essential that a common time-frame for first follow-up is agreed on. Two weeks, as in our study, is probably too short to observe the full benefit of FCM. Some authors have measured the increase in Hb level 6 weeks after infusion of FCM. Since the major rationale for FCM is a rapid correction of anaemia, this time-period appears a bit long. We suggest for future studies a compromise period of 4 weeks to assess the effectiveness of FCM.
The most important feature of FCM is that it is designed to mimic physiological ferritin. It is Ph neutral[5],[6],[7] and has physiological osmolarity. It has a good safety profile, and serious adverse events are rare (<1 in 200 000). Our findings are in line with the known safety profile of FCM.
Infusion of FCM is expected not only to rapidly correct anaemia but also to restore body iron reserves. The clinical utility of FCM would be enhanced if it were to replenish body iron reserves over an extended period. We measured the serum ferritin level as a marker of body iron reserves at different time-points. As a group, the median serum ferritin level at second follow-up (i.e. at the time of delivery) suggests that body iron reserves were replenished. Yet, 12 of 62 (19.4%) pregnant females were moderately anaemic at the time of delivery. One possible explanation is that at least for some females the dose of iron was inadequate. We had capped the maximum dose as a single infusion of 1000 mg of iron even if the calculated dose of iron required was higher. The recommended treatment is to give a second dose at least a week later to make up the remainder of the calculated body iron deficit. Future effectiveness studies may administer the full calculated dose of iron and then assess its impact at the time of delivery.
Strengths and limitations
Unlike other studies from India which recruited a mixed group of patients, we restricted the recruitment to moderate-to-severe anaemic pregnant females. Hence, the study population was well-defined. We had an a priori hypothesis and an adequate sample size so that the study was sufficiently powered to detect the mean difference in Hb levels. The laboratory procedures were performed by an experienced and qualified person. Therefore, the study was assured of internal validity. Whether the findings would hold true to other attendees of ANC clinics in different contexts cannot be commented upon.
The number of participants at baseline, first follow-up (day 15) and second follow-up (at the time of delivery) were 77, 63 and 62, respectively [Figure - 1]. Of the 14 participants who did not attend the first follow-up on day 15, 8 returned at the time of their delivery; between the first and second follow-ups, an additional 9 females were lost to follow-up, and hence the second follow-up was possible for a total of 62 participants. Thus, 6 participants who did not return for the subsequent second or third follow-ups could have most probably gone to their parental homes for delivery, which is a social practice in the study area. For the 9 participants who were lost to follow-up between the first and second follow-ups, the most probable reason could be that they chose to deliver elsewhere. For these 9 participants, the mean (SD) values of Hb at baseline were 9 (0.9) g/dl, and at the first follow-up 10.3 (1.1) g/dl.
Conclusion
Infusion of FCM not only rapidly corrected anaemia but also had a sustained effect which maintained Hb to normal levels till the time of delivery. It replenished body iron reserves. FCM is safe and effective in treating anaemia in pregnant females in the second and third trimester at the subdistrict healthcare setting in India.
FCM should be considered as an option for treating moderate-to-severe anaemia among pregnant females when rapid increase in Hb level is desired. It could be administered in any health facility that has a medical doctor, and capacity to deal with the unlikely event of an adverse reaction.
Conflicts of interest. None declared
| 1. | International Institute for Population Sciences (IIPS) and Macro International. National Family Health Survey (NFHS-3), 2005–06. Vol. 2. India, Mumbai:International Institute for Population Sciences; 2007. [Google Scholar] |
| 2. | Allen LH. Anemia and iron deficiency: Effects on pregnancy outcome. Am J Clin Nutr 2000;71:1280S–4S. [Google Scholar] |
| 3. | Development WHOD of N for H and. Iron Deficiency Anaemia: Assessment, Prevention and Control: A Guide for Programme Managers; 2001. Available at www.who.int/iris/handle/10665/66914 (accessed on 13 Dec 2016). [Google Scholar] |
| 4. | Devasenapathy N, Singh R, Moodbidri P, Bhushan H, Gupta S, Zodpey SP, et al. An observational study on the use of IV iron sucrose among anaemic pregnant women in government healthcare facilities from two states of India. J Obstet Gynaecol India 2015;65:230–5. [Google Scholar] |
| 5. | Patel J, Patel K, Patel J, Sharma A, Date S. Comparison of intravenous iron sucrose and ferric carboxymaltose therapy in iron deficiency anemia during pregnancy and postpartum period. J Pharm Sci Biosci Res 2015;5:239–43. [Google Scholar] |
| 6. | StataCorp. Stata Statistical Software: Release 12. College Station, TX:StataCorp LP; 2011. [Google Scholar] |
| 7. | Mishra V, Chaudhary S, Gandhi K, Sharma U, Patel U. Study of intravenous ferric carboxy maltose in iron deficiency anemia during pregnancy and post-partum period––Safety and efficacy. Indian J Obstet Gynecol Res 2015;2:69–72. [Google Scholar] |
| 8. | Mishra V, Dharaiya N, Aggarwal R, Choudhary S, Gandhi K. Study of intravenous ferric carboxy maltose in iron deficiency anemia in women attending gynecological clinic-safety and efficacy. Int J Reprod Contracept Obstet Gynecol 2015;4:968–71. [Google Scholar] |
| 9. | Verma U, Singh S, Chandra M, Chandra M, Garg R, Singh S, et al. To evaluate the efficacy and safety of single dose intravenous iron carboxymaltose verses multidose iron sucrose in postpartum cases of severe iron deficiency anemia. Int J Reprod Contracept Obstet Gynecol 2015;4:442–6. [Google Scholar] |
| 10. | Rathod S, Samal SK, Mahapatra PC, Samal S. Ferric carboxymaltose: A revolution in the treatment of postpartum anemia in Indian women. Int J Appl Basic Med Res 2015;5:25–30. [Google Scholar] |
| 11. | Naqash A, Ara R, Bader GN. Effectiveness and safety of ferric carboxymaltose compared to iron sucrose in women with iron deficiency anemia: Phase IV clinical trials. BMC Womens Health 2018;18:6. [Google Scholar] |
| 12. | Sharma N, Thiek JL, Natung T, Ahanthem SS. Comparative study of efficacy and safety of ferric carboxymaltose versus iron sucrose in post-partum anaemia. J Obstet Gynaecol India 2017;67:253–7. [Google Scholar] |
| 13. | Moore RA, Gaskell H, Rose P, Allan J. Meta-analysis of efficacy and safety of intravenous ferric carboxymaltose (Ferinject) from clinical trial reports and published trial data. BMC Blood Disord 2011;11:4. [Google Scholar] |
Fulltext Views
1,834
PDF downloads
434




