Translate this page into:
Non-adherence to CML therapy and its clinical implications in India
Corresponding Author:
Hari Menon
Department of Medical Oncology (Leukemia and Lymphoma), Tata Memorial Hospital, Dr E. Borges Road, Parel, Mumbai 400012, Maharashtra
India
harimenondls@gmail.com
| How to cite this article: Menon H. Non-adherence to CML therapy and its clinical implications in India. Natl Med J India 2017;30:142-147 |
Abstract
Clinical trials have shown that early and deeper cytogenetic/ molecular responses to tyrosine kinase inhibitors (TKIs) help in achieving improved long-term outcomes including lower rates of disease progression in chronic myeloid leukaemia (CML). However, the level of molecular responses achieved with TKI therapy in patients with CML is variable and this could be explained by differences in adherence to CML therapy. A systematic literature review of CML studies reporting adherence to BCR–ABL inhibitors from the USA, Asia and Europe (19 articles: 9 retrospective, 4 prospective, rest cross-sectional) showed that average adherence varies from 19% to 100% of the proportion of prescribed drug taken. Some factors that contribute to non-adherence include patient attitudes, adverse events associated with therapy, treatment complexities and socioeconomic issues. This article focuses on the problem of non-adherence to therapy in CML, especially from an Indian perspective, and offers suggestions for its mitigation.
Introduction
Chronic myeloid leukaemia (CML) is a haematopoietic stem cell disease characterized by the presence of a specific chromosome (Philadelphia chromosome) and its corresponding molecular marker BCR–ABL fusion transcripts (tyrosine kinase proteins). The management of CML was revolutionized by the advent of targeted therapy, the tyrosine kinase inhibitors (TKIs). Imatinib was the first TKI to be approved for CML in 2001, followed by more potent BCR–ABL inhibitors, nilotinib and dasatinib. All three drugs are now approved for initial therapy of CML. BCR–ABL fusion transcripts provide a suitable tool to monitor the disease burden in CML. Studies have shown that the prognosis is linked to cytogenetic and molecular responses and achieving specific degrees of disease reduction at specific time points is an essential part of CML management.[1] The success of TKI treatment is determined by continuous optimal dosing and strict treatment adherence. Imatinib boosts the overall survival at 8 years to 85% in patients adhering to treatment.[2] Clinical trials have shown that early and deeper cytogenetic/molecular responses to TKIs help in achieving early undetectable BCR–ABL1 transcripts resulting in improved long-term outcomes including lower rates of disease progression. In the landmark IRIS (International Randomized Study of Interferon and STI571) study, patients who achieved major cytogenetic response (MCyR) by 3 months had a longer time to disease progression during the subsequent 12 months.[3] In another study, the probability of achieving complete cytogenetic response (CCyR) or major molecular response (MMR) decreased steadily with time if CCyR was not achieved by 3, 6 or 12 months.[4] The National Comprehensive Cancer Network (NCCN) Guidelines recommend achievement of BCR–ABL1 transcript level ≤10% as the 3-month treatment response goal.[5],[6] Thus, adherence is crucial in the first 18 months of treatment as achieving a deep level of response within this timeframe produces better long-term outcomes. Moreover, to maintain the response, patients need prolonged period of treatment. Long-term IRIS follow-up studies have shown that imatinib's therapeutic benefits extend up to 7 years and longer in patients with CML who get uninterrupted treatment.[7]
Approximately 40% of patients who remain on imatinib for more than 5 years will have undetectable minimal residual disease (UMRD).[8] Therefore, continued compliance throughout life is of utmost importance for better clinical outcomes. The European LeukemiaNet management guidelines for CML recommend continuing TKI indefinitely in responding patients, even in those with UMRD.[9]
Implications of Poor Adherence on Disease Outcomes
With the current recommendation of ‘indefinite or lifelong TKI therapy in CML for maximum benefits’ issues related to treatment adherence become particularly important. Adherence is defined as the extent to which patients are able to follow the recommendations for prescribed treatments.[10],[11] Studies of adherence in patients with non-cancer medical conditions (diabetes, hypercholesterolemia, hypertension) requiring chronic medications have shown that adherence to medications is poor (20% to 50%) and about half the patients discontinue therapy within the first 6 months of treatment.[12] It is a misconception that cancer, because of its grave severity, may be associated with better adherence to therapy. Non-adherence to BCR–ABL inhibitors in CML is common; studies have shown that non-adherence in CML is a serious problem, with a quarter to one-third of patients being non-adherent.[10],[11] Non-adherence is associated with unfavourable outcomes including suboptimal treatment responses, imatinib resistance, disease relapse and increase in healthcare costs [Table - 1].[13],[14],[15],[16] In the Hammersmith Hospital study, Marin et al. reported that patients with ≤90% adherence were significantly less likely to achieve MMR or complete molecular response (CMR) than were patients with >90% adherence. No molecular responses were observed when adherence was ≤80% (p<0.001).[17] Patients whose imatinib doses were increased were found to have poor adherence (86. 4%); adherence was the only independent predictor for inability to achieve an MMR (RR 17.66, p=0.006).[17] Poor adherence may lead to loss of treatment response. In one study, patients who had ≤85% adherence to imatinib were significantly more likely to lose CCyR at 2 years and experience treatment failure than patients with >85% adherence.[15] Studies evaluating adherence to second-line BCR–ABL TKIs are few. Dasatinib with its once daily regimen may have better compliance compared to nilotinib, which is taken twice daily. In a retrospective study published in 2012, patients receiving second-line nilotinib had poorer adherence compared to those taking dasatinib (100 mg once daily). No correlation was found between adherence and treatment response.[18]

In India, there are few published studies on assessment of treatment adherence in CML. In a retrospective analysis using the Glivec International Patient Assistance Program (GIPAP) database, 29.6% of patients were not completely adherent to imatinib and, in a multivariate analysis, non-adherence was the only factor significantly affecting event-free survival (EFS). The overall estimated 5-year EFS rate was 70.8% (95% CI 63.3–78.3) with a median follow-up of 39 months. The 5-year EFS rate in adherent patients was 76.7% compared to 59.8% (p=0.011) in non-adherent patients, pointing to the impact of adherence on survival.[19] An analysis of unpublished data from Tata Memorial Centre, Mumbai among 1074 patients with CML (year 2004–09) showed that almost 26% of patients receiving Glivec through an access programme were non-adherent to therapy. However, the non-adherence was much higher among those receiving Glivec through an access programme compared to those receiving generic imatinib, indicating that factors other than cost are key contributors to non-adherence. It was also seen that overall treatment interruptions were far higher in those who did not achieve a CCyR (37.7%, n=242) as compared to those who did (21.4%, n=832) (personal communication).
Measuring Adherence to Therapy
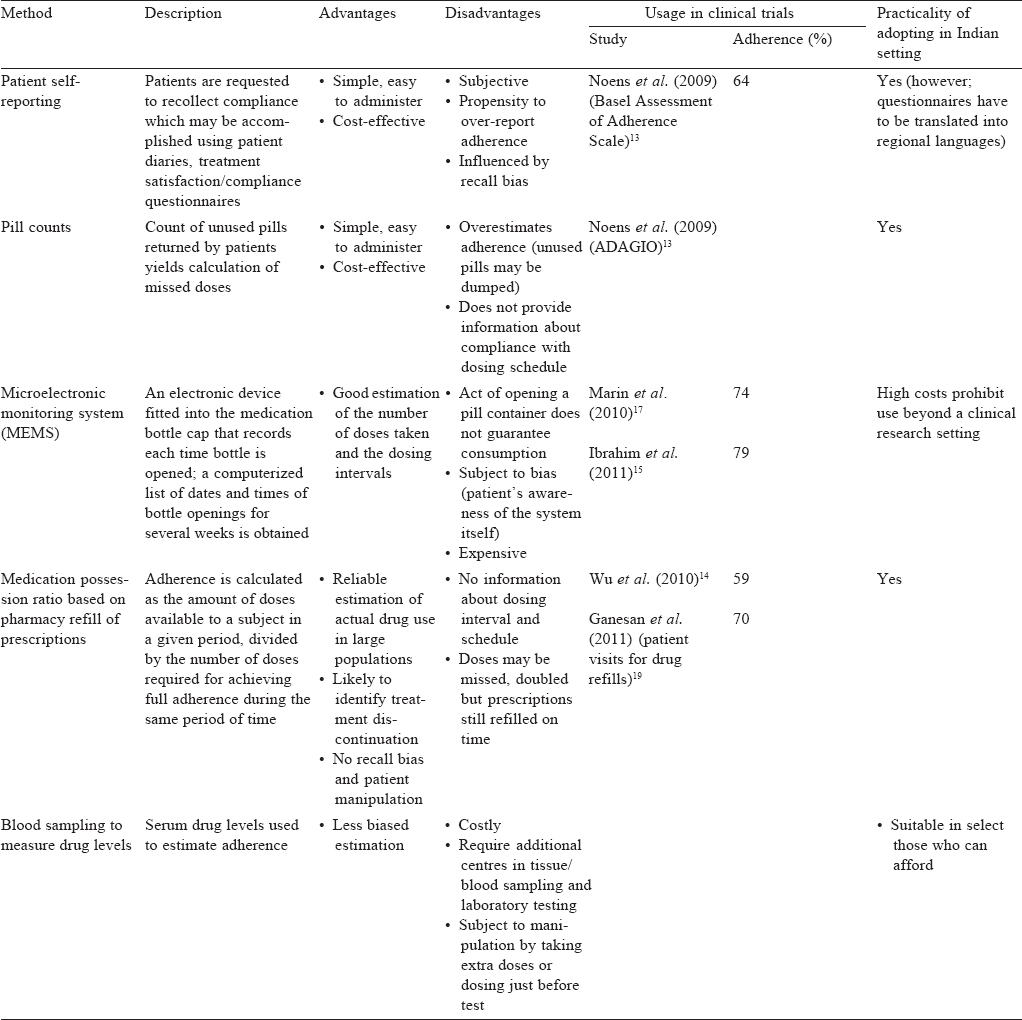
There are several methods to measure adherence though none of them is absolutely accurate. The ADAGIO study showed that adherence rates vary with different assessment methods. Some commonly used methods of treatment adherence, their advantages and disadvantages, practicality of application in India are discussed in [Table - 2].
Factors Affecting Adherence to Cml Therapy
A variety of factors may affect treatment adherence. Patient-related factors include poor memory, forgetfulness, difference in perception levels and lack of understanding treatment instructions. In India high rates of illiteracy and poor education may affect cognitive levels and patients may not be able to fully comprehend instructions of drug usage or understand them wrongly. In the Ganesan et al. study, more than half the patients had poor understanding about the illness and planned treatment.[19] Patients may hesitate to ask pertinent questions or seek more knowledge about the disease if a physician is unapproachable or less communicative. In India, treatment centres are busy and overcrowded; physicians may not be able to allocate sufficient time to patients to answer all their questions satisfactorily.
Non-adherence is also determined by patient attitudes. Some may lose motivation and discontinue treatment, some lack faith in benefits of complete treatment while some may get disillusioned with the side-effects. Younger patients may have lower adherence rates. In the report by Marin et al., the median age for patients with an adherence rate ≤90% was 43.8 years compared with 53.8 years for patients with adherence rate >90% (p=0.004).[17] In another retrospective analysis, women were nearly twice as likely as men to have interruption in treatment (p=0.009).[20]
A strong predictor for non-adherence is adverse events (AEs), which have been consistently implicated in illnesses requiring long-term treatment. AEs may range from minor inconveniences to debilitating conditions. The most commonly reported AEs (grades 1–2 severity) with imatinib are oedema, muscle cramps, diarrhoea, nausea, rash, fatigue and joint pains while more severe AEs (grades 3–4) include haematological events and elevated liver enzymes. The comparison of nilotinib and imatinib as firstline therapy in the ENESTnd (Evaluating Nilotinib Efficacy and Safety in clinical Trials—newly diagnosed patients) study revealed lower rates of nausea, diarrhoea, vomiting, muscle spasm and oedema with nilotinib compared with imatinib while rates of rash, headache and alopecia were higher with nilotinib. Neutropenia was lower with nilotinib while thrombocytopenia and anaemia rates were comparable.[21] Similarly, comparison of first-line therapy of dasatinib or imatinib in the DASISION (DASatinib versus Imatinib Study In treatment-Naive CML patients) study showed that non-haematological AEs were more frequent with imatinib than with dasatinib. Pleural effusion was seen in 10% of dasatinib-treated patients and none in imatinib users.[22] These AEs affect quality of life and often lead to intentional non-adherence where patients decide to miss doses to avoid experiencing AEs. In a study, patients with CML were asked to report current symptoms and their interference with daily activities. Of a total of 44 symptoms reported, most frequent were fatigue, pain and nausea. These symptoms interfered with daily activities in at least 30% of patients, leading some patients to stop or contemplate stopping treatment, or to decrease the dose/frequency of treatment.[23] Significantly lower adherence rates were reported by Marin et al. in patients with AEs such as asthenia, nausea, muscle cramps, bone or joint pains.[17]
Treatment complexities such as specific dietary restrictions and dosing requirements affect adherence. Once daily dosing (imatinib, dasatinib) offers an advantage over twice daily dosing (nilotinib). Nilotinib is affected by food and must be taken on an empty stomach, which requires patients not to eat for at least 2 hours before and 1 hour after taking the drug. In a survey on understanding the overall treatment burden and satisfaction among patients with CML, treatment difficulty was higher among users of nilotinib (63.3%) than imatinib (19.2%) and was least with dasatinib (2.6%) users (p<0.0001). Nilotinib users reported more missed doses (mean [SD] 1.02 [1.60]) than imatinib users (0.45 [1.08]; p<0.05).[24] The presence of comorbid conditions requiring other medications also affect adherence. In Darkow et al.'s analysis, adherence decreased as concomitant medications increased (p=0.002) and treatment interruptions were more in patients with a high cancer complexity (p=0.03).[20]
Perhaps the biggest of all predictors of adherence are socioeconomic factors. In India, most patients cannot afford the high cost of treatment. In a typical middle class or a lower middle class family, patients may prefer family needs or responsibilities over their therapy. They may either discontinue treatment once they experience some clinical benefit or may restrict doses until they can manage sufficient funds to afford further therapy.
Strategies to Enhance Adherence
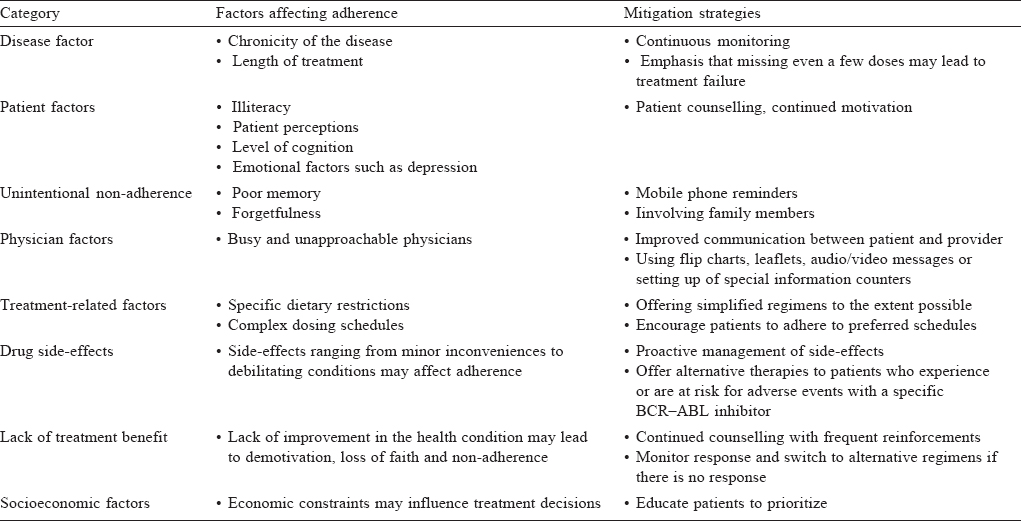
Treatment adherence in chronic illnesses is a complex issue and is affected by many factors. Understanding the reasons for non-adherence in a patient helps decide what strategy works best for each individual and to provide tailored interventions.
Non-adherence due to patient attitude
Non-adherence due to patients' attitude is often difficult to change. Healthcare professionals play an important role in encouraging adherence. Improved patient–physician rapport, spending sufficient time with patients and caregivers explaining various aspects of the disease, educating them to recognize and immediately report AEs, prompt and appropriate management of the AEs help improve adherence.[10] Importance of treatment adherence and how every single ‘mis sed dose’ may impact progres s should be driven home. In overcrowded medical centres, clinicians often may not be able to allot sufficient time but it is worth remembering that a few extra minutes in the initial visits go a long way in saving time and healthcare costs due to potential negative clinical outcomes associated with non-adherence. An effective and economical solution would be to involve the healthcare team who could educate patients using flip charts, leaflets, audio/video messages in waiting areas or to set up special counters to provide adequate information to patients and clarify their doubts.
Unintentional non-adherence
Unintentional non-adherence due to forgetfulness can be overcome with support from family members who could successfully help incorporate medications into a patient's routine. Mobile phones are often used in interventions to increase adherence;[11] with the wide penetration of mobile phones in most remote villages, setting alarms or SMS for administration reminders helps in achieving better compliance.
Monitoring of response
Monitoring treatment response is important in CML. Unexplained increases in BCR–ABL1 transcript levels, loss of response to treatment as determined by blood counts or cytogenetic testing, or disease relapse marked by the manifestation of clinical symptoms are all signs of waning adherence and/or inadequate response to treatment. In Marin et al.‘s series, 10/23 (44%) patients with unexplained increases had adherence rates ≤90%, whereas only 10/64 (16%) with no significant change in transcript levels had an adherence rate ≤90% (p=0.01).[17] Timely monitoring helps identify decreased adherence allowing early interventions to improve adherence. The NCCN guidelines recommend molecular monitoring of BCR–ABL1 transcript levels once every 3 months and evaluation for potential adherence problems when expected treatment response is not achieved at 3, 12 and 18 months. In India, there are few laboratories with facilities for molecular testing though some major cancer institutes are now trying to set up their own testing facilities.
Management of AEs
AEs of CML therapy may affect day-to-day activities and often lead to lowered adherence. Physicians and the healthcare team should reassure patients that AEs may be effectively managed and encourage patients not to stop taking their medication when AEs become uncomfortable. Physicians should proactively manage AEs. The NCCN guidelines provide specific recommendations for the management of AEs associated with TKIs. While AEs of grades 1–2 severity are often managed with supportive therapy, more severe AEs (grades 3–4) may require interruption of treatment followed by resumption at the original or reduced dose. Cytopenias are seen in a large number of patients on TKI therapy. Frequent monitoring of blood counts (weekly in the first month, monthly during the second and third months and every 3 months thereafter) is recommended. Patients should be educated to recognize related signs and symptoms (fever, infections and easy bruising) and report immediately. Peripheral oedema is another bothersome complication associated with TKIs. Treatment for moderate oedema includes close electrolyte monitoring, salt restriction, low-dose loop diuretics and potassium–magnesium supplements while severe oedema often necessitates treatment interruption.
Gastrointestinal (GI) AEs during TKI therapy include increased frequency of bowel movements in the initial treatment period but tend to return to normal after a few weeks. One way to tackle GI symptoms is to take TKIs with water and large meals (except nilotinib, which has to be taken in a fasting state). Some TKIs are associated with rare and serious AEs (nilotinib: peripheral arterial disease; dasatinib: pulmonary arterial hypertension), which may necessitate permanent discontinuation or switching to an alternative TKI. The NCCN guidelines provide detailed guidance on managing the AEs of TKI; in addition, individual prescribing information of each of these drugs should be referred for guidance.
Socioeconomic factors
Economic constraints may influence treatment decisions with patients often preferring family needs over their own. Non-adherence is associated with negative consequences which would mean a further financial burden; educating patients and caregivers about this possibility would help prioritize therapy above other needs.
[Table - 3] summarizes the factors affecting adherence and some mitigation strategies for non-adherence.
Stopping Imatinib: A Realistic Goal?
Recently, researchers have been examining the possibility of discontinuing TKI therapy in select patients who achieve a stable molecular response. The Stop Imatinib (STIM) study was a prospective study to assess whether imatinib can be discontinued in patients with CMR. The results showed that in a subset of 69 patients with at least 12 months follow-up (median 24 months), molecular relapse occurred in 42 (61%) patients (40 before 6 months) though these patients responded to the reintroduction of imatinib (26 achieved CMR after imatinib rechallenge). Further, patients with imatinib therapy for at least 50 months had a 53% likelihood of molecular relapse, whereas this rate was 78% in patients with a shorter duration of treatment. Results of the STIM study were backed by similar evidence from the Australian TWISTER study. Persistence of CMR even after discontinuation in 41% (95% CI 29–52) of patients in the STIM study indicated the possibility of safely discontinuing imatinib in select patients. However, stopping imatinib is still not endorsed by international guidelines; currently, indefinite TKI therapy remains the preferred standard of care and discontinuation of therapy is only advised in the context of a clinical trial.[25],[26]
Conclusion
Although BCR–ABL TKIs have brought an unprecedented change in the outlook of patients with CML, monitoring and ensuring adherence to these agents is paramount in achieving optimal clinical benefits. Patient education, holistic healthcare approach encompassing drug therapy, behavioural counselling, improved patient–physician communication, early and aggressive management of AEs, strong family support and understanding are all strategies that may help to increase treatment adherence and help in optimizing TKI therapy.
Acknowledgements
Professional medical writing and editorial assistance was provided by Dr Manisha Ginde and Dr Kavitha Rangappa at DiagnoSearch Life Sciences (P) Ltd., and was funded by Bristol-Myers Squibb.
Conflict of interest. None declared. The author did not receive financial compensation for writing the manuscript.
| 1. | Morotti A, Fava C, Saglio G. Milestones and monitoring. Curr Hematol Malig Rep 2015;10:167–72. [Google Scholar] |
| 2. | Deininger M, O'Brien S, Guilhot F, Goldman J, Hochhaus A, Hughes T, et al. International randomised study of interferon vs STI571 (IRIS) 8-year follow up: Sustained survival and low risk for progression or events in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) treated with imatinib. Blood: 51st Annual Meeting of the American Society of Hematology. New Orleans, LA: USA; 5-8 Dec 2009: 114,22:462 (Abstract 1126). [Google Scholar] |
| 3. | Kantarjian HM, O'Brien S, Cortes JE, Shan J, Giles FJ, Rios MB, et al. Complete cytogenetic and molecular responses to interferon-α-based therapy for chronic myelogenous leukemia are associated with excellent long-term prognosis. Cancer 2003;97:1033–41. [Google Scholar] |
| 4. | Hehlmann R, Hanfstein B, Erben P, Lauseker M, Fabarius A, Schnittger S, et al. The prognostic significance of early molecular and cytogenetic response for long-term progression-free and overall survival in imatinib-treated chronic myeloid leukemia (CML) J Clin Oncol 2012;30 (Suppl):ASCO abstract 6510. [Google Scholar] |
| 5. | Baccarani M, Deininger MW, Rosti G, Hochhaus A, Soverini S, Apperley JF, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood 2013;122:872–84. [Google Scholar] |
| 6. | National Comprehensive Cancer Network. Clinical practice guidelines in oncology (NCCN Guidelines®) Chronic myelogenous leukemia version 3.2014 [Internet]. NCCN.org. Available at http://williams.medicine.wisc.edu/cml.pdf (accessed on 6 Jan 2017). [Google Scholar] |
| 7. | O'Brien SG, Guilhot F, Goldman DM, Hochhaus A, Hughes TP, Radich JP, et al. International randomised study of interferon versus STI571 (IRIS) 7-year follow-up: Sustained survival, low rate of transformation and increased rate of major molecular response in patients with newly diagnosed chronic myeloid leukemia in chronic phase treated with imatinib. Blood 2008;112: Abstract 186. [Google Scholar] |
| 8. | Branford S, Seymour JF, Grigg A, Arthur C, Rudzki Z, Lynch K, et al. BCR–ABL messenger RNA levels continue to decline in patients with chronic phase chronic myeloid leukemia treated with imatinib for more than 5 years and approximately half of all first-line treated patients have stable undetectable BCRABL using strict sensitivity criteria. Clin Cancer Res 2007;13:7080–5. [Google Scholar] |
| 9. | Baccarani M, Cortes J, Pane F, Niederwieser D, Saglio G, Apperley J, et al.; European LeukemiaNet. Chronic myeloid leukemia: An update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol 2009; 27:6041–51. [Google Scholar] |
| 10. | Jabbour E, Saglio G, Radich J, Kantarjian H. Adherence to BCR–ABL inhibitors: Issues for CML therapy. Clin Lymphoma Myeloma Leuk 2012;12:223–9. [Google Scholar] |
| 11. | Hugtenburg JG, Timmers L, Elders PJ, Vervloet M, van Dijk L. Definitions, variants, and causes of nonadherence with medication: A challenge for tailored interventions. Patient Prefer Adherence 2013;7:675-82. doi: 10.2147/PPA.S29549. [Google Scholar] |
| 12. | Kripalani S, Yao X, Haynes RB. Interventions to enhance medication adherence in chronic medical conditions. Arch Intern Med 2007;167:540–50. [Google Scholar] |
| 13. | Noens L, van Lierde MA, De Bock R, Verhoef G, Zachée P, Berneman Z, et al. Prevalence, determinants,and outcomes of nonadherence to imatinib therapy in patients with chronic myeloid leukemia: The ADAGIO study. Blood 2009;113: 5401–11. [Google Scholar] |
| 14. | Wu EQ, Guerin A, Yu AP, Bollu VK, Guo A, Griffin JD. Retrospective real-world comparison of medical visits, costs, and adherence between nilotinib and dasatinib in chronic myeloid leukemia. Curr Med Res Opin 2010;26:2861–9. [Google Scholar] |
| 15. | Ibrahim AR, Eliasson L, Apperley JF, Milojkovic D, Marco Bua L, Szydlo LR, et al. Poor adherence is the main reason for loss of CCyR and imatinib failure for chronic myeloid leukemia patients on long-term therapy. Blood 2011;117:3733–6. [Google Scholar] |
| 16. | Noens L, Hensen M, Kucmin-Bemelmans I, Lofgren C, Gilloteau I, Vrijens B. Measurement of adherence to BCR–ABL inhibitor therapy in chronic myeloid leukemia: Current situation and future challenges. Haematologica 2014;99:437–47. [Google Scholar] |
| 17. | Marin D, Bazeos A, Mahon FX, Eliasson L, Milojkovic D, Bua M, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol 2010;28:2381–8. [Google Scholar] |
| 18. | Yood MU, Oliveria SA, Cziraky M, Hirji I, Hamdan M, Davis C. Adherence to treatment with second-line therapies, dasatinib and nilotinib, in patients with chronic myeloid leukemia. Curr Med Res Opin 2012;28:213-19; Curr Med Res Opin 2012;28:1164; author reply 1164-65. doi: 10.1185/03007995.2011.649849 [Google Scholar] |
| 19. | Ganesan P, Sagar TG, Dubashi B, Rajendranath R, Kannan K, Cyriac S, et al. Nonadherence to imatinib adversely affects event free survival in chronic phase chronic myeloid leukemia. Am J Hematol 2011 ;86:471^·. [Google Scholar] |
| 20. | Darkow T, Henk HJ, Thomas SK, Feng W, Baladi JF, Goldberg GA, et al. Treatment interruptions and non-adherence with imatinib and associated healthcare costs: A retrospective analysis among managed care patients with chronic myelogenous leukaemia. Pharmacoeconomics 2007;25:481–96. [Google Scholar] |
| 21. | Saglio G, Kim DW, Issaragrisil S, le Coutre P, Etienne G, Lobo C, et al.; ENESTnd Investigators. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med 2010;362:2251–9. [Google Scholar] |
| 22. | Kantarjian H, Shah NP, Hochhaus A, Cortes J, Shah S, Ayala M, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. NEngl J Med 2010;362:2260–70. [Google Scholar] |
| 23. | Williams LA, Ault P, Cleeland CS, Reynolds RJ, Shah NA, Shah PK, et al. Symptom burden in chronic myeloid leukemia (CML). ASCO Annual Meeting Proceedings. J Clin Oncol 2010;28 (Suppl 1):6133. [Google Scholar] |
| 24. | Hirji I, Gupta S, Goren A, Chirovsky DR, Moadel AB, Olavarria E, et al. Chronic myeloid leukemia (CML): Association of treatment satisfaction, negative medication experience and treatment restrictions with health outcomes, from the patient's perspective. Health Qual Life Outcomes 2013;11:167. [Google Scholar] |
| 25. | Mahon FX, Réa D, Guilhot J, Guilhot F, Huguet F, Nicolini F; Intergroupe Français des Leucémies Myéloïdes Chroniques. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: The prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol 2010;11:1029–35. [Google Scholar] |
| 26. | Barton MK. Discontinuation of imatinib may be possible in chronic myelogenous leukemia. CA: A Cancer Journal for Clinicians 2011;61:65-6. Version of Record online: 4 Mar 2011 | DOI: 10.3322/caac.20110. [Google Scholar] |
Fulltext Views
1,458
PDF downloads
468




