Translate this page into:
Population-based estimate of urinary stones from Ballabgarh, northern India
2 Department of Microbiology, All India Institute of Medical Sciences, Ansari Nagar, New Delhi 110029, India
Corresponding Author:
Shashi Kant
Centre for Community Medicine, All India Institute of Medical Sciences, Ansari Nagar, New Delhi 110029
India
skant76@gmail.com
| How to cite this article: Lohiya A, Kant S, Kapil A, Gupta SK, Misra P, Rai SK. Population-based estimate of urinary stones from Ballabgarh, northern India. Natl Med J India 2017;30:198-200 |
Abstract
Background. Stones in the urinary tract are a common condition but there is paucity of data on their population-based estimates in India. We describe our findings of the burden of urinary stones during a cross-sectional study with another primary goal.Methods. We conducted the study at Ballabgarh Health and Demographic Surveillance System, Haryana, among residents aged 18 years or above. We used simple random sampling to enrol participants. Self-reported history of urinary stones was elicited through an interview schedule. Results of the descriptive analysis were described as proportions with 95% confidence intervals (CI) or as mean wherever applicable. Bivariate analysis was done using t-test and chi-square test as applicable.
Results. The response rate for our study was 86.6%; lifetime prevalence (95% CI) of urinary stones was 7.9% (5.7, 10.8). In a majority of participants, urinary stones were diagnosed at an age of 20–40 years (55.9%), mostly by an ultrasonography examination (94.1%).
Conclusions. A high burden of urinary stones is indicated in the working-age population in northern India at the community level. Untreated urinary stones can lead to an acute emergency (colic) or may have long-term adverse consequences, e.g. hydronephrosis, which have implications for the healthcare delivery system.
Introduction
Urinary stones occur commonly and are often associated with complications.[1] The condition affects the working-age population.[2] Studies providing population-based estimates of the burden of urinary stones have been done mostly in western countries.[2] We did not find any population-based study that provided the burden of urinary stones from India. There is also evidence of changes in the epidemiology of the disease.[3] We did a study primarily to assess the magnitude of resistance among urinary isolates of Escherichia coli and Klebsiellapneumoniae against various classes of antibiotics in an apparently healthy population of northern India. The presence of urinary stones was explored in the study as a risk factor for the presence of Enterobacteriaceae in the urine. We share this incidental finding.
Methods
We conducted this study in the Ballabgarh Health and Demographic Surveillance System (HDSS) from November 2012 to December 2013.[4] It is situated in Ballabgarh block of district Faridabad, Haryana. All residents 18 years or older, willing to participate in the studywere included. The sample size was calculated for the primary objective of the study. If we assume the prevalence of urinary stones to be 8.8%,[5] absolute precision to be 3%, level of significance as 5%, power as 80% and response rate to be 80%, then the reassessed sample size was 430. Hence, a sample of 500 participants in the parent study would be adequate for this study as well.
We did simple random sampling to select 500 participants using the health management and information system (HMIS) database of Ballabgarh HDSS. The HMIS is a computerized database of all individuals residing in the Ballabgarh HDSS. At least two house visits were made to contact all eligible participants.
A semi-structured, pre-tested interview schedule was used to collect sociodemographic information, history regarding urinary stones and other clinical variables. The presence of urinary stones was ascertained by asking for current and/or past history of stones anywhere in the urinary tract. The diagnosis of stones in the urinary tract was self-reported.
Data entry was done in EpiInfo 3.5.4 (Center for Disease Control, USA) and statistical analysis was done using Stata 11 (Stat Corp College Station, Texas, USA). Results of the descriptive analysis were described as proportions with 95% CI or as means wherever applicable. Bivariate analysis was done using the t-test and chi-square test as applicable. Ethical approval was obtained from the ethics committee of All India Institute of Medical Sciences, New Delhi. Written informed consent was taken from all participants and confidentiality of data was maintained.
Results
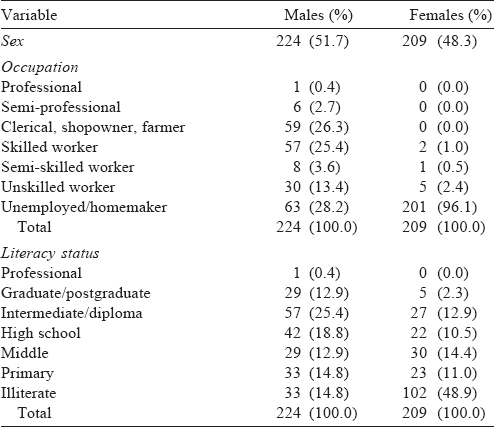
Of the 500 participants selected for the study, information could be obtained from 433 (response rate 86.6%). Almost equal proportions of participants were males (51.7%) and females (48.3%). The mean (SD) age of male and female participants was 38.1 (1.1) and 39.1 (1.2) years, respectively. Most of the participants were educated till intermediate/diploma among males (25.4%) and were illiterate among females (48.9%). Other demographic characteristics of the participants were similar [Table - 1].
Prevalence of urinary stones
The lifetime prevalence (95% CI) of urinary stones in the study participants was 7.9% (5.7%–10.8%). Most participants (61.8%) had urinary stones in the past with no symptoms suggestive of urinary stones at present. Diagnosis was established on the basis of ultrasonography examination in most participants (94.1%). Mean (SD) age at the time of diagnosis of urinary stones was 37.6 (15.7) years. Most participants (55.9%) were diagnosed with urinary stones at 20–40 years of age [Table - 2].

Presenting symptoms of urinary stones
The occupation of the participants, history of burning micturition in the past year, history of painful micturition in the past year, and any history of urinary retention in the past were significantly associated (p<0.05) with the presence of stones in the urinary tract [Table - 3].

Discussion
This incidental finding gives an estimate of the burden of urinary stones at the community level in northern India. Ours is possibly the first study providing an estimate of urinary stones at the population level in India. However, population-based estimates for the burden of urinary stones are available from other countries.[2] Most studies done outside India have estimated the prevalence of urinary stones to be 4% to 15% among the general population.[2] The prevalence of urinary stones in our study was comparable with estimates from other countries. The observed differences in the estimates could be because of different methodologies followed. Few studies have shown an increase in the prevalence of urinary stones over the years in western countries.[6],[7],[8] Many dietary and environmental factors have been found to be responsible for this.[3]
The prevalence of urinary stones was more among males than females. The male to female ratio for the prevalence of urinary stones was found to be 1.5:1, which is similar to that in other studies.[2] There was a significant association between the presence of urinary stones and that of burning micturition, painful micturition in the past year and ever history of urinary retention. Many previous studies have described that the presence of urinary tract infection increases the chances of urinary stone formation and the presence of urinary stones is itself a risk factor for the development of urinary tract infection.[9]
Urinary stones are associated with significant morbidity and are also responsible for investigations, surgical interventions, hospitalizations, as well as loss of wages.[10] Thus, it imposes a burden on the health system of the country and family of the affected individuals.[10] Urinary stones are associated with complications that further increase the financial burden on the family and affect the quality of life.[1] Urinary stones commonly affect the age group of 20–40 years, which is an economically productive population.[5]
The presence of urinary stones in this study was self-reported. Thus, only those who had clinical symptoms and were investigated for urinary stones were likely to be included. Those with asymptomatic stones would have been missed. Even symptomatic individuals, who did have urinary stones but were not investigated by ultrasonography, would have been missed. This would have resulted in under-estimating the burden of urinary stones. Moreover, some participants with urinary stones would go undetected by ultrasonography because of its low sensitivity.[11] Hence, our estimate of urinary stones is likely to be a conservative estimate of the actual prevalence.
The data were collected by a single investigator, thus eliminating inter-observer bias. This study has a few other limitations. Self- reported history of urinary stones was used for establishing diagnoses. Since this was part of another study, the dietary and environmental factors associated with the presence of urinary stones were not explored. Hence, we are unable to comment on their role on the prevalence of urinary stones. This is a cross- sectional study, hence temporality of association with known and unknown risk factors could not be established.
In conclusion, the present study provides information on the prevalence of urinary stones in northern India. Since this condition mainly affects the working-age population, these findings have implications for our public health system.
Acknowledgements
We acknowledge the study participants and the multipurpose health workers of the primary health centres of Dayalpur and Chhainsa, Haryana who facilitated data collection.
| 1. | Domingos F, Serra A. Nephrolithiasis is associated with an increased prevalence of cardiovascular disease. Nephrol Dial Transplant 2011 ;26:864-8. [Google Scholar] |
| 2. | Romero V, Akpinar H, Assimos DG. Kidney stones : A global picture of prevalence, incidence, and associated risk factors. Rev Urol 2010;12:e86-96. [Google Scholar] |
| 3. | Roudakova K, Monga M. The evolving epidemiology of stone disease. Indian J Urol 2014;30:44-8. [Google Scholar] |
| 4. | Kant S, Misra P, Gupta S, Goswami K, Krishnan A, Nongkynrih B, et al. The Ballabgarh Health and Demographic Surveillance System (CRHSP-AIIMS). Int J Epidemiol 2013;42:758-68. [Google Scholar] |
| 5. | Scales CD, Smith AC, Hanley JM, Saigal CS. Urologic Diseases in America Project. Prevalence of kidney stones in the United States. Eur Urol 2012;62:160-5. [Google Scholar] |
| 6. | Stamatelou KK, Francis ME, Jones CA, Nyberg LM, Curhan GC. Time trends in reported prevalence of kidney stones in the United States: 1976-1994. Kidney Int 2003;63:1817-23. [Google Scholar] |
| 7. | Hesse A, Brändle E, Wilbert D, Köhrmann K-U, Alken P. Study on the prevalence and incidence of urolithiasis in Germany comparing the years 1979 vs. 2000. Eur Urol 2003;44:709-13. [Google Scholar] |
| 8. | Trinchieri A, Coppi F, Montanari E, Del Nero A, Zanetti G, Pisani E. Increase in the prevalence of symptomatic upper urinary tract stones during the last ten years. Eur Urol 2000;37:23-5. [Google Scholar] |
| 9. | Miano R, Germani S, Vespasiani G. Stones and urinary tract infections. Urol Int 2007;79 Suppl 1:32-6. [Google Scholar] |
| 10. | Shuster J, Scheaffer RL. Economic impact of kidney stones in white male adults. Urology 1984;24:327-31. [Google Scholar] |
| 11. | Fowler KAB, Locken JA, Duchesne JH, Williamson MR. US for detecting renal calculi with nonenhanced CT as a reference standard. Radiology 2002;222:109-13. [Google Scholar] |
Fulltext Views
3,599
PDF downloads
1,755




