Translate this page into:
Role of closure of patent foramen ovale in cryptogenic stroke: Current status
Corresponding Author:
B Sunil Abhishek
Department of Cardiology, Vydehi Institute of Medical Sciences and Research Centre, # 82, EPIP Area, Whitefield, Bengaluru 560066, Karnataka
India
sunil167@yahoo.com
| How to cite this article: Abhishek B S, Bhambhani A. Role of closure of patent foramen ovale in cryptogenic stroke: Current status. Natl Med J India 2017;30:268-271 |
Abstract
Ischaemic stroke is among the leading causes of disability and death. Despite extensive vascular, cardiac and serological evaluations, the cause remains unknown in 20%-40% of patients. These are classified as cryptogenic stroke. Paradoxical embolism is one of the many causes of cryptogenic stroke. The term paradoxical embolism is used to describe an embolus of venous origin entering the systemic circulation through a patent foramen ovale (PFO), atrial septal defect (ASD), ventricular septal defect or extracardiac communication such as pulmonary arteriovenous malformation. PFO is present in about 25% of the population. PFO is seen more commonly in patients with cryptogenic stroke. Secondary prevention of stroke in such patients includes the prevention of formation of a thrombus with antiplatelets, vitamin K antagonists or closure of the PFO by either surgery or the percutaneous route. Percutaneous closure using devices has been shown to be safe and beneficial in preventing secondary stroke. Data from randomized trials have shown device closure to be superior to medical management in the secondary prevention of cryptogenic stroke due to PFO.
Introduction
Ischaemic stroke is among the leading causes of disability and death.[1],[2] The classification used in the TOAST trial (Trial of ORG10172 in Acute Stroke Treatment)[3] categorizes acute stroke, according to aetiology, into those due to large artery atherosclerosis, cardioembolism, small vessel occlusion, other determined aetiology and stroke of undetermined aetiology. Despite extensive vascular, cardiac and serological evaluations, the aetiology remains unexplained in 20%–40% of patients. These are classified as cryptogenic stroke.[4],[5],[6],[7] Several pathophysiological mechanisms have been proposed for cryptogenic stroke such as paroxysmal atrial fibrillation, paradoxical embolism due to patent foramen ovale (PFO) or atrial septal abnormalities, thrombophilias and autoimmune and inflammatory states. Paradoxical embolism is defined as one where the thrombus originates in the systemic venous circulation and enters the systemic arterial circulation through a PFO, atrial septal defect (ASD), ventricular septal defect or extracardiac communication such as pulmonary arteriovenous malformation. In 1877, Cohnheim coined the term paradoxical embolism to describe an embolism of venous origin entering the systemic circulation through a PFO.[8] The embolus can originate in the lower extremities, pelvic veins, in an atrial septal aneurysm (ASA) or from a clot around the edges of PFO.
Foramen ovale with its flap-like valve between the right and left atria is an important component of foetal circulation. At birth, the flap is closed functionally due to relative increase in the left atrial pressure, and later structurally due to adhesions. In approximately 25% of individuals, the foramen ovale remains patent and is a potential source of right-to-left shunting. Autopsy studies indicate an incidence of 15%–35% of PFO in the general population.[9],[10],[11],[12] The incidence of PFO tends to decrease with increasing age.[11] Even transient shunting across PFO, such as during early ventricular systole, Valsalva manoeuvre and repetitive cough, can lead to a paradoxical embolism.
Detection of PFO in vivo
A number of ultrasound-based modalities, including transthoracic echo (TTE), transoesophageal echo (TEE),[13],[14] transcranial Doppler[15],[16] and transmitral Doppler,[17] can be used to diagnose PFO. The detection of a PFO by TEE using agitated saline as contrast and colour Doppler imaging both at rest and with manoeuvres has correlated well with autopsy studies.[18] TEE is the most sensitive technique for detecting PFO. TEE allows visualization of the atrial septum flap covering the ASD and also the transient right-to-left shunt occurring in early ventricular systole or during straining.
PFO in Cryptogenic Stroke
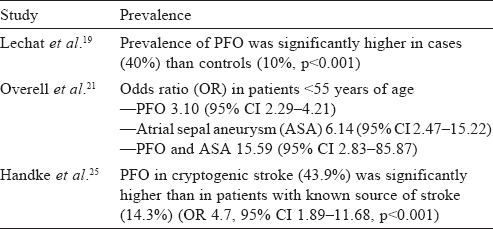
The association of PFO with cryptogenic stroke was initially observed by Lechat et al.[19] and Webster et al.[20] A large meta-analysis of case-control studies summarized the evidence that PFO, ASA or both are more likely to be found in patients with stroke than in stroke-free individuals.[21] Another study in 2002, evaluating the benefit of secondary prevention by giving aspirin in patients of cryptogenic stroke and PFO, found a similar positive correlation.[22] However, a population-based study in 2006, evaluating the relation between PFO and cerebrovascular ischaemic events, found no evidence.[23] In this study, PFO was found in only 13% of the stroke group when compared with an average of 32% in other studies. Later studies confirmed the association of PFO with cryptogenic stroke in patients younger than 55 years. PFO showed a 4-fold increase in prevalence in patients of cryptogenic stroke when compared with controls (~45% v. 11%, p<0.001).[24] A prospective study of 503 patients found a significant prevalence of PFO in cryptogenic stroke in young (<55 years) and old patients.[25] The presence of ASA increases the risk of recurrence even more [Table - 1]. In patients older than 55 years, initial studies have been inconclusive.[26],[27],[28],[29] A large TEE study done in 2007 confirmed the association of PFO and ischaemic stroke in those >55 years of age.[25] Overall, PFO is associated significantly more frequently with cryptogenic stroke in the young (<55 years old), but this difference is found to be less pronounced in the old (>55 years old).
Secondary Prevention
Therapeutic measures for secondary prevention of ischaemic stroke in patients with PFO are focused mainly on prevention of thrombus formation by antiplatelets, antithrombotics and/or prevention of paradoxical embolism through the PFO by surgery or the transcatheter route. Medical management usually involves giving vitamin K antagonists, targeting an international normalized ratio (INR) of 2–3 and/or antiplatelets. Despite being on medical management, patients with PFO and cryptogenic stroke have an increased risk of recurrence.[22] The risk of recurrent stroke in a 4-year period was found to be 25%[30] in patients on medical therapy. An ASA along with PFO further increases the risk of recurrence. In such a setting, closure of the PFO is an attractive option to prevent recurrence of stroke. A pooled analysis of data available till 2004 showed a modest benefit from percutaneous closure when compared to medical management with the combined rate of recurrent stroke, death or transient ischaemic attack of 2.95 events/100 person-years, slightly lower than that of the medical management group.[31] Death and major complications such as major haemorrhage and tamponade occurred in about 1.5% of patients in a meta-analysis of 13 5 5 patients.[18] Other complications such as atrial arrhythmias, device arm fractures, device embolization and device thrombosis occurred in 7.9% of patients.[18] Improvements in the technology and quality of devices, along with experience with the procedure, have decreased complications and the rate of recurrence. A recent meta-analysis showed that the incidence of atrial fibrillation was higher in the device group compared to medical management (odds ratio [OR] 3.29, 95% CI 0.86-12.6, I2=60%).[32] However, after excluding the CLOSURE I study, which used STAR flex septal closure system, there was a statistically insignificant incidence (OR 1.81, 95% CI 0.60–5.42, I2=4%). The incidence of major bleeding also was not different between the two groups (OR 1.43, 95% CI 0.47–4.42, I2=49%).[32]
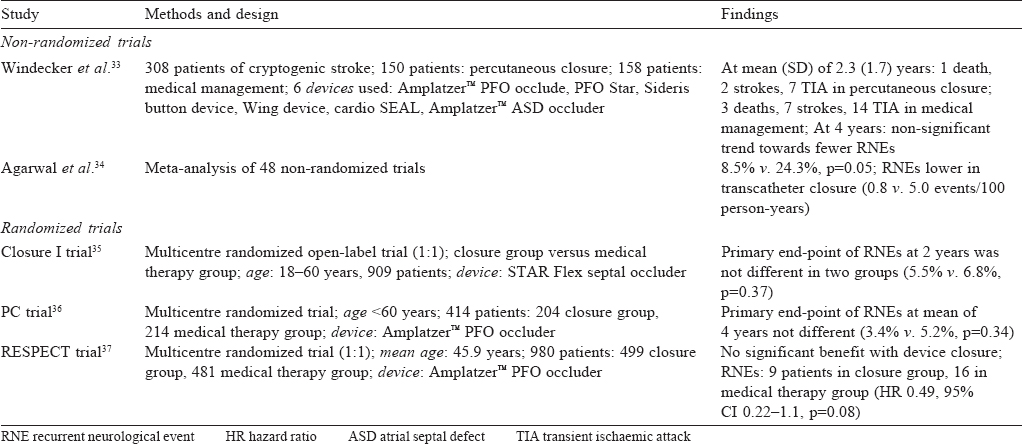
Windecker et al.[33] did a non-randomized trial comparing medical management (vitamin K antagonists or antiplatelet agents) in 158 patients with percutaneous device closure in 150 patients who underwent PFO device closure and found the latter to be as effective as medical management. In patients who had complete closure of the PFO, after 2 years it was more beneficial than medical management.
Agarwal et al.[34] did a meta-analysis of the available nonrandomized observational studies comparing transcatheter closure with medical therapy for PFO in the prevention of recurrent neurological events (RNEs). This analysis found device closure to be better in the prevention of recurrence.
The CLOSURE I trial (2012)[35] was the first randomized controlled trial that compared the STAR flex septal closure system to medical management in patients with cryptogenic stroke and PFO. It failed to show any benefit of device closure. In fact, there were increased adverse events in the device closure arm— a high prevalence of residual shunt and high incidence of device-related atrial fibrillation.
The PC (2013)[36] and RESPECT (2013)[37] trials used the Amplatzer™ PFO septal occluder. Both these studies showed a benefit of device closure, but the difference was not statistically significant [Table - 2]. These studies had a low incidence of strokes compared to the normal population and more dropouts; factors that may have contributed to the results. However, the benefits on mortality and morbidity were more apparent on long-term follow-up (after 2 years) and this continued after 4 years.[36],[37]
In 2013, Khan et al. did a systematic review and meta-analysis of the three available randomized trials (CLOSURE I, PC and RESPECT). It showed that device closure was beneficial and had a 33%–39% reduction in the hazard of RNEs. When only the RESPECT and PC trials were analysed (using Amplatzer™ PFO occluder), the reduction in recurrence was 46%–58%.[32]
The 10-year follow-up results of the RESPECT trial presented at the 28th Transcatheter Therapeutics showed a significant benefit with closure of PFO in patients <60 years of age and a cryptogenic stroke.[38] The mean follow-up for the device closure group was 6.3 years and for the medical management group 5.5 years. In the intention-to-treat cohort, there was a 45% relative risk reduction (hazard ratio [HR] 0.55, 95% CI 0.305–0.999, p=0.046) in recurrent ischaemic stroke for the PFO group and a 62% risk reduction (HR 0.38, 95% CI 0.18–0.79, p=0.007) of recurrent ischaemic stroke due to unknown mechanism. An additional sensitivity analysis of all-cause stroke in patients <60 years of age showed a 58% relative risk reduction (HR 0.42, 95% CI 0.21–0.83, p=0.01). The procedure-related serious adverse events (SAEs) were cardiac perforation (needing pericardiocentesis) 0.4%, cardiac perforation (requiring no treatment) 0.4%, access site bleeding 0.6%, right atrial thrombus 0.2%, deep venous thrombosis 0.2%, atrial fibrillation 0.2%, and others (allergic responses, vasovagal response) 0.4%. Device-related SAEs were ischaemic stroke 0.4%, pulmonary embolism 0.4%, thrombus in the right atrium (not attached to device) 0.2%, explant/surgical intervention 0.4%, residual shunt 0.2%, and others (chest tightness, atrial flutter, non-sustained ventricular tachycardia and sepsis) 0.8%.
Based on the results of the extended follow-up of the RE SPECT trial, the US Food and Drug Administration (FDA) approved Amplatzer™ PFO occluder for prevention of recurrent stroke in the age group of 18–60 years with cryptogenic stroke, presumed to be due to paradoxical embolism as determined by a neurologist and a cardiologist following an evaluation to rule out the known causes of ischaemic stroke. The US FDA panel opined that the benefits outweigh the risks. However, the American Academy of Neurologists has advised against routine use of device closure in PFO with cryptogenic stroke and has emphasized that patients need to be counselled regarding the condition, and the commonality and rarity of it being the cause of cryptogenic stroke.
At the 3rd European Stroke Organization Conference in May 2017, evidence from trials such as Gore REDUCE and CLOSE were presented.[39] The Gore REDUCE study is a multicentre randomized controlled trial which compared long-term outcomes with closure by GORE HELEX septal occluder to the outcomes with medical management alone. The trial included 664 patients who were randomized in a 2:1 ratio to device closure and medical management groups. The annualized recurrent stroke rate was 0.39 in the device closure group and 1.70 in the medical management group (HR 0.23, 95% CI 0.09–0.62). The CLOSE trial, done in Germany and France, enrolled 663 patients and randomized them to (i) closure of PFO; (ii) oral anticoagulant therapy; or (iii) oral antiplatelet therapy. Comparison between PFO closure and antiplatelet therapy showed four recurrent strokes in the antiplatelet group compared with none in the closure group (HR 0.03, 95% CI 0–0.25, p<0.001). An interesting observation was that patients with PFO and ASA had more recurrent stroke (2%) than patients with PFO and a large shunt (0.5%).
Conclusion
PFO is a congenital abnormality found in 15%–35% of individuals.[9],[10],[11],[12] It is associated with stroke of undetermined origin/ cryptogenic stroke in about 32% of individuals in various studies.[23] Percutaneous device closure is a safe and attractive option for secondary prevention of cryptogenic stroke. Randomized trials have shown survival benefit in patients undergoing device closure compared with medical management. Awareness of this condition and the treatment options available need to be improved and also must be communicated clearly to patients. A combined team of a cardiologist and a neurologist such as a heart–brain team seems to be an innovative option for a correct decision on an individual basis and to avoid unnecessary procedures. Multicentric trials are needed to further validate the benefit of device closure and to formulate clear guidelines for patients of cryptogenic stroke with PFO.
Conflicts of interest. None
| 1. | Pandian JD, Sudhan P. Stroke epidemiology and stroke care services in India. J Stroke 2013;15:128-34. [Google Scholar] |
| 2. | Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. ; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2013 update: A report from the American Heart Association. Circulation 2013;127:e6-e245. [Google Scholar] |
| 3. | Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke 1993;24: 35-41. [Google Scholar] |
| 4. | Grau AJ, Weimar C, Buggle F, Heinrich A, Goertler M, Neumaier S, et al. Risk factors, outcome, and treatment in subtypes of ischemic stroke: The German stroke data bank. Stroke 2001;32:2559-66. [Google Scholar] |
| 5. | Kolominsky-Rabas PL, Weber M, Gefeller O, Neundoerfer B, Heuschmann PU. Epidemiology of ischemic stroke subtypes according to TOAST criteria: Incidence, recurrence, and long-term survival in ischemic stroke subtypes : A population-based study. Stroke 2001;32:2735-40. [Google Scholar] |
| 6. | Schulz UG, Rothwell PM. Differences in vascular risk factors between etiological subtypes of ischemic stroke: Importance of population-based studies. Stroke 2003; 34:2050-9. [Google Scholar] |
| 7. | Amarenco P, Bogousslavsky J, Caplan LR, Donnan GA, Hennerici MG. New approach to stroke subtyping: The A-S-C-O (phenotypic) classification of stroke. Cerebrovasc Dis 2009;27:502-8. [Google Scholar] |
| 8. | Cohnheim J. Thrombose und emboli. Vorl Allg Pathol 1877;1:134. [Google Scholar] |
| 9. | Penther P. Patent foramen ovale: An anatomical study. Apropos of 500 consecutive autopsies. Arch Mal Coeur Vaiss 1994;87:15-21. [Google Scholar] |
| 10. | Schroeckenstein RF, Wasenda GJ, Edwards JE. Valvular competent patent foramen ovale in adults. Minn Med 1972;55:11-13. [Google Scholar] |
| 11. | Hagen PT, Scholz DG, Edwards WD. Incidence and size of patent foramen ovale during the first 10 decades of life: An autopsy study of965 normal hearts. Mayo Clin Proc 1984;59:17-20. [Google Scholar] |
| 12. | Sweeney LJ, Rosenquist GC. The normal anatomy of the atrial septum in the human heart. Am Heart J 1979;98:194-9. [Google Scholar] |
| 13. | Pinto FJ. When and how to diagnose patent foramen ovale. Heart2005;91:438-40. [Google Scholar] |
| 14. | Di Tullio M, Sacco RL, Venketasubramanian N, Sherman D, Mohr JP, Homma S. Comparison of diagnostic techniques for the detection of a patent foramen ovale in stroke patients. Stroke 1993;24:1020-4. [Google Scholar] |
| 15. | Job FP, Ringelstein EB, Grafen Y, Flachskampf FA, Doherty C, Stockmanns A, et al. Comparison of transcranial contrast Doppler sonography and transesophageal contrast echocardiography for the detection of patent foramen ovale in young stroke patients. Am J Cardiol 1994;74:381-4. [Google Scholar] |
| 16. | Kerr AJ, Buck T, Chia K, Chow CM, Fox E, Levine RA, et al. Transmitral Doppler : A new transthoracic contrast method for patent foramen ovale detection and quantification. J Am Coll Cardiol 2000;36:1959-66. [Google Scholar] |
| 17. | Schneider B, Zienkiewicz T, Jansen V, Hofmann T, Noltenius H, Meinertz T. Diagnosis of patent foramen ovale by transesophageal echocardiography and correlation with autopsy findings. Am J Cardiol 1996;77:1202-9. [Google Scholar] |
| 18. | Khairy P, O’Donnell CP, Landzberg MJ. Transcatheter closure versus medical therapy of patent foramen ovale and presumed paradoxical thromboemboli: A systematic review. Ann Intern Med 2003;139:753-60. [Google Scholar] |
| 19. | Lechat P, Mas JL, Lascault G, Loron P, Theard M, Klimczac M, et al. Prevalence of patent foramen ovale in patients with stroke. N Engl J Med 1988;318:1148-52. [Google Scholar] |
| 20. | Webster MW, Chancellor AM, Smith HJ, Swift DL, Sharpe DN, Bass NM, et al. Patent foramen ovale in young stroke patients. Lancet 1988;2:11-12. [Google Scholar] |
| 21. | Overell JR, Bone I, Lees KR. Interatrial septal abnormalities and stroke: A metaanalysis of case-control studies. Neurology 2000;55:1172-9. [Google Scholar] |
| 22. | Homma S, Sacco RL, Di Tullio MR, Sciacca RR, Mohr JP; PFO in Cryptogenic Stroke Study (PICSS) Investigators. Effect of medical treatment in stroke patients with patent foramen ovale: Patent foramen ovale in Cryptogenic Stroke Study. Circulation 2002;105:2625-31. [Google Scholar] |
| 23. | Petty GW, Khandheria BK, Meissner I, Whisnant JP, Rocca WA, Christianson TJ, et al. Population-based study of the relationship between patent foramen ovale and cerebrovascular ischemic events. Mayo Clin Proc 2006;81:602-8. [Google Scholar] |
| 24. | Di Tullio MR, Homma S. Patent foramen ovale. AHA/ASA guidelines and statements handbook. Dallas, Texas, USA:American Heart Association/American Stroke Association; 2009. [Google Scholar] |
| 25. | Handke M, Harloff A, Olschewski M, Hetzel A, Geibel A. Patent foramen ovale and cryptogenic stroke in older patients. N Engl J Med 2007;357:2262-8. [Google Scholar] |
| 26. | de Belder MA, Tourikis L, Leech G, Camm AJ. Risk of patent foramen ovale for thromboembolic events in all age groups. Am J Cardiol 1992;69:1316-20. [Google Scholar] |
| 27. | Di Tullio M, Sacco RL, Gopal A, Mohr JP, Homma S. Patent foramen ovale as a risk factor for cryptogenic stroke. Ann Intern Med 1992;117:461-5. [Google Scholar] |
| 28. | Hausmann D, Mügge A, Becht I, Daniel WG. Diagnosis of patent foramen ovale by transesophageal echocardiography and association with cerebral and peripheral embolic events. Am J Cardiol 1992;70:668-72. [Google Scholar] |
| 29. | Jones EF, Calafiore P, Donnan GA, Tonkin AM. Evidence that patent foramen ovale is not a risk factor for cerebral ischemia in the elderly. Am J Cardiol 1994;74:596-9. [Google Scholar] |
| 30. | Mas JL, Arquizan C, Lamy C, Zuber M, Cabanes L, Derumeaux G, et al. Recurrent cerebrovascular events associated with patent foramen ovale, atrial septal aneurysm, or both. N Engl J Med 2001;345:1740-6. [Google Scholar] |
| 31. | Homma S, Sacco RL. Patent foramen ovale and stroke. Circulation 2005; 112: 1063-72. [Google Scholar] |
| 32. | Khan AR, Bin Abdulhak AA, Sheikh MA, Khan S, Erwin PJ, Tleyjeh I, et al. Device closure of patent foramen ovale versus medical therapy in cryptogenic stroke: A systematic review and meta-analysis. JACC Cardiovasc Interv 2013;6:1316-23. [Google Scholar] |
| 33. | Windecker S, Wahl A, Nedeltchev K, Arnold M, Schwerzmann M, Seiler C, et al. Comparison of medical treatment with percutaneous closure of patent foramen ovale in patients with cryptogenic stroke. J Am Coll Cardiol 2004;44:750-8. [Google Scholar] |
| 34. | Agarwal S, Bajaj NS, Kumbhani DJ, Tuzcu EM, Kapadia SR. Meta-analysis of transcatheter closure versus medical therapy for patent foramen ovale in prevention of recurrent neurological events after presumed paradoxical embolism. JACC Cardiovasc Interv 2012;5:777-89. [Google Scholar] |
| 35. | Furlan AJ, Reisman M, Massaro J, Mauri L, Adams H, Albers GW, et al. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med 2012;366:991-9. [Google Scholar] |
| 36. | Meier B, Kalesan B, Mattle HP, Khattab AA, Hildick-Smith D, Dudek D, et al. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med 2013;368:1083-91. [Google Scholar] |
| 37. | Carroll JD, Saver JL, Thaler DE, Smalling RW, Berry S, MacDonald LA, et al. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med 2013;368:1092-100. [Google Scholar] |
| 38. | Maxwell YL. Final RESPECT data support PFO closure for cryptogenic stroke prevention. 1 November 2016. Available at www.tctmd.com/news//final-respect-data-support-pfo-closure-cryptogenic-stroke-prevention (accessed on 1 Jun 2017). [Google Scholar] |
| 39. | Hughes S. Finally, success reducing recurrent stroke with PFO closure. 17 May 2017. Available at www.medscape.com/viewarticle/880102#vp_3 (accessed on 1 Jun 2017). [Google Scholar] |
Fulltext Views
2,718
PDF downloads
1,019




