Translate this page into:
Selfie fundus imaging: Innovative approach to retinopathy screening
2 Department of Endocrinology, All India Institute of Medical Sciences, Ansari Nagar, New Delhi 110029, India
Corresponding Author:
Pradeep Venkatesh
Dr Rajendra Prasad Centre for Ophthalmic Sciences, All India Institute of Medical Sciences, Ansari Nagar, New Delhi 110029
India
venkyprao@gmail.com
| How to cite this article: Venkatesh P, Kumar S, Tandon N, Takkar B, Praveen P A. Selfie fundus imaging: Innovative approach to retinopathy screening. Natl Med J India 2018;31:345-346 |
Abstract
The currently available methods of screening for diabetic retinopathy (DR) depend on the availability of healthcare professionals and technology. The high prevalence of diabetes in India, and the need to repeatedly screen such patients for DR and treat them, places an enormous economic and logistic burden. We introduce the concept of screening for DR with ‘selfie retinal imaging’ through this report of 3 patients. The patients can themselves capture retinal images and transfer to a grading centre for further deliberation. If incorporated into the current technological revolution of smartphones, this futuristic concept is likely to be of huge benefit in preventing loss of vision due to diabetes.
Introduction
India is the diabetic capital of the world, and diabetes mellitus is an enormous burden on our limited healthcare resources. Diabetic retinopathy (DR) is a serious complication of the disease, and its management is a huge logistic challenge, especially the need for recurrent screening, lasers and intraocular injections.[1] To prevent or to limit DR in its early stages is the ultimate aim both for preventing patient morbidity and from the community perspective. The National Programme for Control of Blindness and Vision 2020 also emphasize on the need for preventive measures before discussing the curative and rehabilitative aspects of the disease.[2]
The main risk factor for the development of DR is the duration of diabetes. There is usually a window period between the diagnosis of diabetes and development of retinopathy.[3],[4] Since retinopathy is asymptomatic in its initial stages, regular screening is recommended for its early detection and initiation of appropriate management. Methods of retinal screening for DR that have been reported in the literature include direct ophthalmoscopy, indirect ophthalmoscopy, fundus photography and telescreening.[4] All these methods need trained individuals for capturing the retinal images. We report the capture of retinal images by patients using hand-held cameras. Such an approach to retinal screening has not been described earlier to the best of our knowledge.
The Cases
Three patients underwent fundus screening for DR. All these patients were admitted to the endocrinology ward of our institute for control of blood sugar. Cases 1 and 2 had no ocular complaints while Case 3 complained of moderate visual loss. Before the screening procedure, 2 drops of tropicamide (1% solution) were instilled in both eyes for attaining mydriatic photographs.
In all cases, the first ophthalmoscopy and fundus imaging with hand-held fundus camera (Retinal Imaging, Bosch Eye Care Solutions, Germany) were performed in a dark room. Next, the camera was held by the patient himself who clicked photographs after self-focusing, without the aid of the optometrist [Figure - 1]. Thus, ' selfie fundus imaging’ (SFI) was obtained. The photographs were later compared.
Cases 1 and 2 had no DR on either conventional imaging or SFI [Figure - 2] and [Figure - 3]. Case 3 had macular oedema with non-proliferative retinopathy, images of which were adequately captured on both conventional and SFI [Figure - 4].
 |
| Figure 1: Procedure of selfie fundus imaging: Case 1 clicking a selfie fundus imaging with the hand-held camera. Closing the fellow eye allows easier fixation. |
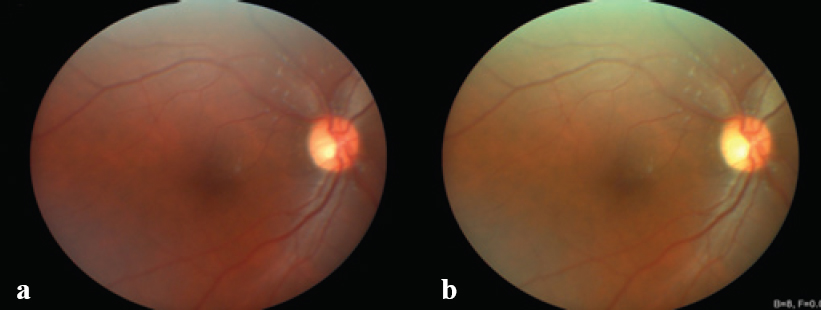 |
| Figure 2: Fundus images of Case 1: (a) clicked by the technician and (b) the selfie fundus imaging clicked by the patient. No diabetic retinopathy is noticeable. |
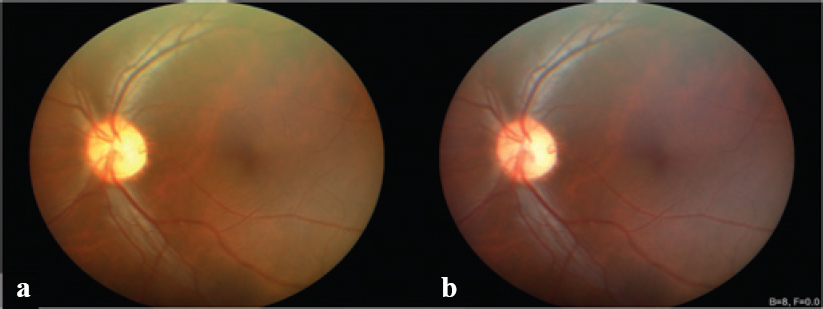 |
| Figure 3: Fundus images of Case 2: (a) clicked by the technician and (b) the selfie fundus imaging clicked by the patient. No diabetic retinopathy is noticeable. |
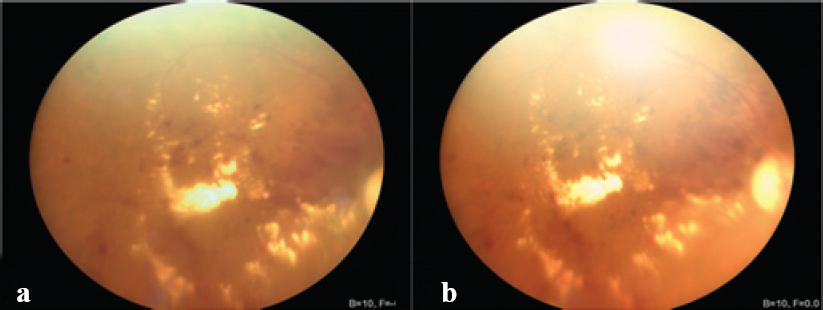 |
| Figure 4: Fundus images of Case 3: (a) clicked by the technician and (b) the selfie fundus imaging clicked by the patient. Macular oedema (yellow-coloured exudates) and retinal haemorrhages are well recognized in both images. |
Discussion
The present methods of screening for DR include ophthalmoscopy by an ophthalmologist and fundus imaging (hand-held or otherwise) by a technician. While ophthalmoscopy is highly sensitive and specific,[5] there is the issue of availability of an ophthalmologist and also lack of pictorial documentation for future comparisons. Two-dimensional fundus imaging gives a magnified view and allows easier documentation of images. Hand-held cameras have further advantages as the patient need not travel far and the resource can be easily brought to the patient’s doorstep by the technician. These images can then be shared by telemedicine,[4] thus obviating travel-related issues for the patient. However, there is still dependence on the technician.
Lately, smartphone enabled hand-held cameras have been approved by the US Food and Drug Administration for retinal imaging.[6] Utilizing in-built applications in smartphones, fundus imaging of reasonable quality has been brought to an entirely new level of inexpensive healthcare.[7],[8] In fact, images can partially be evaluated by automatic techniques also without manual interpretation.[9] Such ocular imaging has been put to use for other diseases as well.
SFI has been tried as early as the 1970s with a Kowa RC-2 fundus camera.[10] It was introduced for monitoring retinal blood circulation during space flight using analogue film-based imaging. The images were obtained in a dark room without the use of mydriatic drops with a high-speed light while customized bite plates and in-built cross-hairs were used for effective fixation.[10] With advances, the proceedure has transgressed into the new millennium as a new retinal self-imaging system using a Kowa Genesis-D hand-held digital camera and a Black and Decker laser level camera.[11] The camera is capable of attaining images with resolution of up to 2 million pixel. The authors contemplated its use for research purposes such as in spacecraft, aircraft, and tent of a mountaineer physiologist.[11] However, the system requires projecting a laser on a wall to attain nasal adduction required for imaging the posterior pole. To overcome this difficulty, media laboratories have developed ‘eye selfie’ imaging devices which provide fixation cues when proper alignment of the fundus to the camera is obtained.[12] To the best of our knowledge, SFI has not been evaluated as a tool for healthcare at large.
We have shown a potential concept of community eye care with SFI for DR. SFI has the obvious advantage of having the potential to obviate the need for trained healthcare professionals in screening for DR as images can be captured by the patients, stored and easily transferred to a grading facility for interpretation and treatment advice. Follow-ups can be easily scheduled without expensive travel and emergencies can be tackled at the earliest. As this screening tool is at its preliminary level, we depended on a previously available hand-held fundus camera used by the patient himself unaided by anyone else. This camera has a 40° non-my driatic imaging field, 5 mega-pixel sensor, an autofocus module (–11 to +3 dioptres) as well as red-free imaging.
SFI is easy for the patient to learn with minimal training. During SFI using this camera, the patients could see a red circle which acted as a fixation target and thus allowed for obtaining images of the macular region. SFI is likely to be easily reproducible. However, an important limitation to SFI is difficulty in patients with low vision. In Case 3, who had low vision due to macular oedema, SFI was time-consuming as the patient could not see the fixation target easily. Perhaps with further technical advances, this limitation can be minimized as fixation targets can be changed or made brighter. These patients may be screened easily by their caregivers too.
With the increasing penetration of smartphones into most households and rapid evolution in smartphone technology, photography and app-based platforms, we believe that SFI has the ability to make screening for diabetes mellitus simpler for patients. In this series, we have presented images of 3 patients. Its validation as an equally effective tool in comparison to conventional fundus imaging is however needed in a larger cohort of patients at both primary and tertiary levels. Such a study would need image readers masked to each others findings for an effective comparison.
To summarize, this is the first report of SFI as a possible tool for community eye care. Further advances and standardization would make SFI an important tool of screening for DR and its timely detection. This is likely to help in decreasing the burden of diabetes-related blindness.
Conflicts of interest. None declared
| 1. | Keenum Z, McGwin G Jr., Witherspoon CD, Haller JA, Clark ME, Owsley C. Patients’ adherence to recommended follow-up eye care after diabetic retinopathy screening in a publicly funded county clinic and factors associated with follow-up eye care use. JAMA Ophthalmol 2016; 134:1221-8. [Google Scholar] |
| 2. | Laskar NB. Evaluating the cost effectiveness of national program for control of blindness in Jorhat district, India. J Nat Sci Biol Med 2015;6:411-14. [Google Scholar] |
| 3. | Goh JK, Cheung CY, Sim SS, Tan PC, Tan GS, Wong TY. Retinal imaging techniques for diabetic retinopathy screening. J Diabetes Sci Technol 2016;10:282-94. [Google Scholar] |
| 4. | Walton OB 4th, Garoon RB, Weng CY, Gross J, Young AK, Camero KA, et al. Evaluation of automated teleretinal screening program for diabetic retinopathy. JAMA Ophthalmol 2016;134:204-9. [Google Scholar] |
| 5. | Malerbi FK, Morales PH, Farah ME, Drummond KR, Mattos TC, Pinheiro AA, et al. Comparison between binocular indirect ophthalmoscopy and digital retinography for diabetic retinopathy screening: The multicenter Brazilian Type 1 Diabetes Study. DiabetolMetab Syndr 2015;7:116. [Google Scholar] |
| 6. | Haddock L J, Qian C. Smartphone technology for fundus photography. Retin Physician 2015; 12:51, 54-6, 58. [Google Scholar] |
| 7. | Micheletti JM, Hendrick AM, Khan FN, Ziemer DC, Pasquel FJ. Current and next generation portable screening devices for diabetic retinopathy. J Diabetes Sci Technol 2016;10:295-300. [Google Scholar] |
| 8. | Rani PK, Raman R, Sharma V, Mahuli SV, Tarigopala A, Sudhir RR, et al. Analysis of a comprehensive diabetic retinopathy screening model for rural and urban diabetics in developing countries. Br J Ophthalmol 2007;91:1425-9. [Google Scholar] |
| 9. | Besenczi R, Tóth J, Hajdu A. A review on automatic analysis techniques for color fundus photographs. Comput Struct Biotechnol J 2016;14:371-84. [Google Scholar] |
| 10. | Philpott DE, Bailey PF, Harrison G, Turnbill C. Modification of a Kowa RC-2 fundus camera for self-photography without the use of mydriatics. Brain Res Bull 1979;4: 123-5. [Google Scholar] |
| 11. | Ozerdem U. A simple nonmydriatic self-retinal imaging procedure using a Kowa Genesis-D hand-held digital fundus camera. Ophthalmic Res 2009;42:125-7. [Google Scholar] |
| 12. | Swedish T, Roesch K, Lee IH, Rastogi K, Bernstein S, Raskar R. eyeSelfie: Self-directed eye alignment using reciprocal eye box imaging. ACM Trans Graph 2015;34:58. [Google Scholar] |
Fulltext Views
1,714
PDF downloads
408




