Translate this page into:
Spectrum of drug-induced liver injury in a tertiary hospital in southern India
Correspondence to ABRAHAM KOSHY; koshyabe@yahoo.com
To cite: Koshy A, Mahadevan P, Mukkada RJ, Francis JV, Chettupuzha AP, Augustine P. Spectrum of drug-induced liver injury in a tertiary hospital in southern India. Natl Med J India 2022;35:78–81.
Abstract
Background
Anti-tuberculosis drugs are thought to account for about 50% of drugs that cause liver injury in India. We show that the spectrum of drugs is much wider than previously reported.
Methods
We evaluated all patients with unexplained acute liver injury presenting during 2006–2016 using a structured proforma for drug-induced liver injury (DILI). The Roussel Uclaf Causality Assessment Method was used to assess causality.
Results
DILI was found in 143 of 2534 patients with acute liver injury. Nineteen patients had probable ayurvedic DILI. The other common causes of DILI were statins (16 patients) and anti-tuberculosis drugs (11 patients). Eight patients had DILI post-liver transplant. Fluconazole was the most common cause of post-liver transplant DILI. Chronic DILI (abnormal liver function test after 12 months of stopping the suspected drug) was found in 2 patients.
Conclusion
In otherwise unexplained acute liver injury, DILI due to ayurvedic drugs should be sought. DILI should be considered in post-liver transplant patients. Patients with DILI should be monitored for at least 12 months to exclude progression to chronic DILI.
INTRODUCTION
More than 1000 drugs that may produce drug-induced liver injury (DILI) were listed in Liver Tox by the year 2017. About half of them cause only elevation of transaminases, but about one-third cause elevation of bilirubin. Antibiotics including anti-tuberculosis drugs are the most common group of drugs that produce liver injury. A publication suggests that in India, about 50% of DILI are due to anti-tuberculosis drugs.1,2 We systematically identified patients with DILI over a period of 10 years and show that the spectrum of drugs is much wider than previously reported.
METHODS
All patients presenting to the gastroenterology department of VPS Lakeshore Hospital, a tertiary care centre, during 2006– 2016 with unexplained acute liver injury, after investigations mentioned below, were evaluated using a structured proforma for DILI. Acute liver injury was defined as alanine aminotransferase (ALT) >5 × upper limit of normal (ULN) or alkaline phosphatase >2 × ULN. Sixty-six per cent had serum total bilirubin >3 mg/dl.
All patients were negative for anti-hepatitis A virus IgM, hepatitis B surface antigen, anti-hepatitis C virus and anti-hepatitis E virus IgM. None had evidence of biliary abnormality on ultrasound of the abdomen. None had a history of reasonable alcohol intake or current hypotension.
Autoimmune hepatitis (antinuclear antibody and smooth muscle antibody), Wilson disease (K-F ring and serum ceruloplasmin) and other unusual causes of hepatitis were excluded with appropriate tests including liver biopsy where required.
As recommended by an international expert panel of a phenotyping standardization project, the Roussel Uclaf Causality Assessment Method (RUCAM, Table I) was used to assess causality.3–5 Necessary parameters were captured prospectively and all patients were followed up for at least 6 months. An attempt was made to follow up all patients for a further 6 months. In addition, patients with herb (ayurvedic)-induced liver injury (HILI) were also assessed using the Council for International Organizations of Medical Sciences (CIOMS) scale6 (Table II).
| Item | Score |
|---|---|
| Temporal relationship of start of drug to start of illness | |
| Initial treatment 5–90 days; subsequent treatment course 1–15 days | +2 |
| Initial treatment <5 or >90 days; subsequent treatment course >15 days | +1 |
| From cessation of drug: within 15 days; or within 15 days after subsequent treatment | +1 |
| Otherwise | 0 |
| Course | |
| ALT decrease >50% from peak within 8 days | +3 |
| ALT decrease >50% from peak within 30 days | +2 |
| If the drug is continued or ALT decreased <50% from peak >30 days, or inconclusive | 0 |
| Against causative role of drug | –2 |
| Risk factors | |
| Alcohol use, 1; No alcohol use, 0 | 1 or 0 |
| Age >55 years, +1; Age <55 years, 0 | 1 or 0 |
| Concomitant drug | |
| No concomitant drug administered | 0 |
| Concomitant drug with suggestive or compatible time of onset | –1 |
| Concomitant known hepatotoxin with suggestive or compatible time of onset | –2 |
| Concomitant drug with positive rechallenge or validated diagnostic test | –3 |
| Non-drug causes: Six are primary: current hepatitis A, B, C or E, biliary obstruction, acute alcoholic hepatitis (AST <2×ALT), current hypotension (especially if heart disease). Secondary group: Underlying other diseases; possible cytomegalovirus, Ebstein–Barr virus or herpes simplex virus infection In this category, all primary and secondary causes reasonably ruled out | +2 |
| All 7 primary causes ruled out | +1 |
| 5–6 primary causes ruled out | 0 |
| Fewer than 5 primary causes ruled out (maximum negative score for items 4 and 5: –4) | –2 |
| Non-drug cause highly probable | –3 |
| Previous information on hepatotoxicity of the drug in question | |
| Package insert or labelling mention | +2 |
| Published case reports but not in label | +1 |
| Reaction unknown | 0 |
| Rechallenge | |
| Positive (ALT doubles with a drug in question alone) | +3 |
| Compatible (ALT doubles with same drugs as given before initial reaction) | +1 |
| Negative (increase in ALT but <×2 ULN, same condition as when the reaction occurred) | –2 |
| Not done, or indeterminate result | 0 |
ALT alanine aminotransferase ULN upper limit of normal
| Hepatocellular injury | Score | Cholestatic (±hepatocellular) injury | Score | |
|---|---|---|---|---|
|
Time to onset from the beginning of the drug 5–90 days (rechallenge 1–15 days) |
+2 | Rechallenge: 1–90 days | ||
| <5 or >90 days (rechallenge >15 days) | +1 | Rechallenge: >90 days | ||
|
Time to onset from cessation of the drug 15 days (except for slowly metabolized drugs >15 days) |
+1 | 30 days (except for slowly metabolized drugs: >30 days) | ||
|
Course of ALT after cessation of the drug Difference between the peak of ALP and upper limit of normal range |
||||
| Decrease >50% within 8 days | +1 | Decrease>50% within 180 days | +1 | |
| Decrease >50% within 30 days | +2 | Decrease<50% within 180 days | 0 | |
| No information | 0 | Persistence, increase or no information | ||
| Decrease >50% after day 30 Decrease <50% after day 30 or recurrent increase |
0 – 2 |
|||
|
Risk factor ethanol Yes |
+1 | Risk factor ethanol or pregnancy | ||
| No | 0 | |||
|
Risk factor age (years) >55 |
+1 | |||
| <55 | 0 | |||
|
Concomitant drug(s) None or no information |
0 | |||
| Concomitant drug with incompatible time to onset Concomitant drug with compatible or suggestive time to onset Concomitant drug known as hepatotoxin and with compatible or suggestive time to onset |
0 – 1 – 2 |
|||
| Concomitant drug with evidence for its role in this case (positive rechallenge or validated test) | – 3 | |||
| Search for non-drug causes | ||||
| Group I (6 causes) | ||||
| Anti-HAV-IgM, Anti-HBc-IgM/HBV-DNA, Anti-HCV-IgM/ HCV-RNA, hepatobiliary sonography/colour Doppler sonography of liver vessels, alcoholism (AST/ALT >2), acute current hypotension history (particularly if underlying heart disease) | ||||
| Group II | ||||
| Complications of underlying disease(s), Infection suggested by PCR and titre change for CMV (anti-CMV-IgM/IgG), EBV (anti-EBV-IgM/IgG), HSV (anti-HSV-IgM/IgG), VZV (anti- VZV-IgM/IgG) | ||||
|
Evaluation of groups I and II All causes in groups I and II, reasonably ruled out |
+2 | |||
| The 6 causes of group I ruled out | +1 | |||
| 5 or 4 causes of group I ruled out Fewer than 4 causes of group I ruled out Non-drug cause highly probable |
0 – 2 – 3 |
|||
|
Previous information on hepatotoxicity of the drug Reaction labelled in the product characteristics |
+2 | |||
| Reaction published but unlabeled Reaction unknown |
+10 | |||
|
Response to re-administration Doubling of ALT with the drug alone |
+3 | Doubling of ALP with the drug alone | +3 | |
| Doubling of ALT with the drugs already given at the time of first reaction Increase of ALT but less than in the same conditions as for the first administration |
+2 +1 |
Doubling of ALP with the drugs already given at the time of first reaction Increase of ALT but less than in the same conditions as for the first administration |
+2 +1 |
|
| Other situation | 0 | |||
Cholestatic injury: Only differences from hepatocellular injury given. Total points/causality: <0, excluded; 1–2, unlikely; 3–5, possible; 6–8, probable; 8, highly probable ALP alkaline phosphatase ALT alanine aminotransferase AST aspartate aminotransferase CMV cytomegalovirus DDS drugs and dietary supplements EBV Epstein–Barr virus HAV hepatitis A virus HBc hepatitis B core HBV hepatitis B virus HCV hepatitis C virus HSV herpes simplex virus VZV varicella-zoster virus
Chronic DILI was defined as abnormal liver function test (LFT; serum bilirubin, ALT, aspartate aminotransferase [AST] or alkaline phosphatase above the ULN) after 12 months of stopping the suspected drug.8,9 Liver biopsy was performed in 49 patients.
RESULTS
During 2006–2016, a total of 2534 patients with acute liver injury were seen. In 2365, a cause for acute liver injury other than drugs could be found. These 2365 included acute hepatitis A (761), hepatitis B (644), alcoholic hepatitis (605) autoimmune hepatitis (97), hepatitis E (95) divergent other causes (8) and idiopathic (155). The remaining 169 patients were evaluated. Twenty-six were excluded due to incomplete data. The remaining 143 patients were analysed. Seventeen patients had a RUCAM score >8. They were classified as highly probable to have DILI. The drugs implicated in highly probable DILI in more than one patient were anti-tuberculosis drugs, atorvastatin/rosuvastatin, valproate and vincristine. Substances implicated in a single patient as the cause of highly probable DILI were aspirin, carbamazepine, clonazepam, cyclophosphamide, gemcitabine, hair dye, methotrexate, montelukast and nimesulide.
One hundred and twenty-six patients had a RUCAM score between 6 and 8. They were classified as probable DILI. The drugs implicated in probable DILI, in more than two patients, are given in Table III. Of the 19 patients with ayurvedic DILI, 4 had RUCAM score >5 but CIOMS score of 5. All herbal drugs in the study patients were the same as ayurvedic drugs. No patient used dietary herbal supplements or other complementary/ alternative medications. Substances implicated in one or two patients as the cause of probable DILI (not given in the table) were 6-mercaptopurine, aceclofenac, acenocoumarol, azathioprine, azithromycin, captopril, carbamazepine, clopidogrel, cotrimoxazole, cyclophosphamide, cytarabine, domperidone, esomeprazole, etoricoxib, everolimus, febuxostat, ferrous sulphate, gemcitabine/carboplatin, homoeopathic drug, ibuprofen/naproxen, ibuprofen/piroxicam, interferon pegylated, imipramine, isoflurane, lamivudine, lamotrigine, lenalidomide, chlordiazepoxide/trifluoperazine, loratadine, methotrexate, methyldopa, mycophenolate, nimesulide, ofloxacin, ormeloxifene, palonosetron, pantoprazole, penicillin, pentoxifylline, phenytoin, prednisolone, ranitidine, sertraline, sulphasalazine, tacrolimus, tamoxifen and topiramate.
| Drug | Number of patients |
|---|---|
| Ayurvedic drugs | 1 9 |
| Statins | 1 6 |
| Anti-tuberculosis drugs | 1 1 |
| Fluconazole | 6 |
| Amitriptyline | 4 |
| Aspirin | 3 |
| Cephalosporins | 3 |
| Clavulanic acid–amoxicillin | 3 |
| Metformin | 3 |
Nine patients died within 6 months of the onset of DILI; drugs implicated in more than one patient as the cause of death were anti-tuberculosis drugs (3) and ayurvedic drugs (2). Substances implicated in a single patient as the cause of death were aspirin, cyclophosphamide, sulphasalazine and valproate.
Twenty-three patients had DILI and an associated liver disease; associated liver diseases found in more than one patient were status post-liver transplant (8), autoimmune hepatitis (6) and cirrhosis (5). Eight patients were post-liver transplant. In 6 of 8 patients, fluconazole was the probable cause of DILI. On the other hand, all patients with fluconazole as the probable cause of DILI were status post-liver transplant and the type of liver injury was hepatocellular or mixed in all except one with a cholestatic injury. Hepatic artery thrombosis, bile leak and rejection were excluded in all of them. Associated liver diseases found in a single instance were chronic hepatitis C, paucity of bile ducts, HBV carrier status and acute myeloid leukaemia. All patients with associated liver disease were classified as probable DILI. None of these patients died within 6 months of the onset of DILI.
The patterns of liver injury seen were hepatocellular (78), cholestatic (37) and mixed (27). The spectrum for ayurvedic drugs was similar to the general pattern: hepatocellular (13), cholestatic (4) and mixed (3).
Liver biopsy was performed, when the treating physician wanted exclusion of other causes of liver disease and patient consent was available, in 49 patients (29 men). Their mean (SD age was 46 (15) years, and 22 had hepatocellular type of liver injury, 14 had cholestatic and 13 had a mixed injury. The biopsy showed cholestasis in 19, bridging necrosis/fibrosis in 14, steatosis in 10, portal inflammation in 9, ductular proliferation in 7, lobular inflammation in 5, granuloma in 4 and cirrhosis in 2 (with chronic DILI).
Sixty-six patients were followed up for more than 12 months after stopping the suspected drug. Twenty-one of 66 patients had total serum bilirubin, AST, ALT or alkaline phosphatase above the ULN more than 12 months after stopping the suspected drug. Probable cause was attributed to the underlying disease in 15 (liver transplant related 6, autoimmune hepatitis 4, leukaemia 2, diabetes with fatty liver 2 and cirrhosis with activity 1), second episode of DILI in 4 and chronic DILI in 2 patients (Fig. 1). Both patients with chronic DILI were of cholestatic type and at 12 months after stopping the suspected drug. One had serum total bilirubin 2.9 mg/dl, AST 61 i.u./L and alkaline phosphatase 189 i.u./L. The other had serum total bilirubin 3.1 mg/dl, normal AST and alkaline phosphatase 245 i.u./L. Both had normal ALT. One of the 2 patients with chronic DILI had initial liver biopsy showing cholestatic hepatitis.
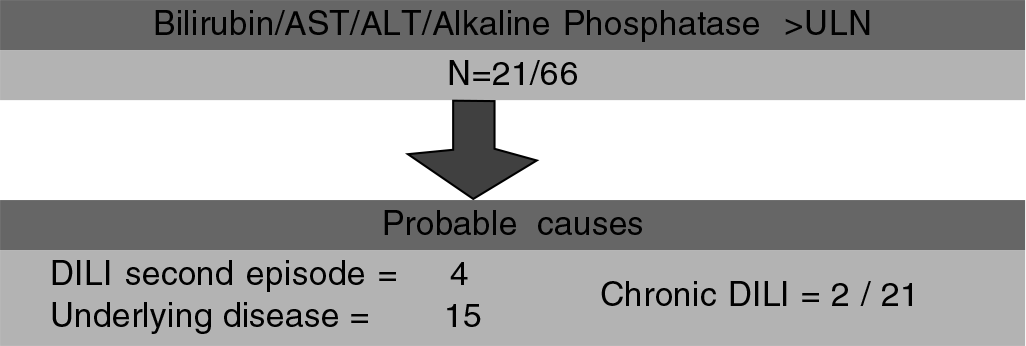
- Flowchart showing probable causes of abnormal liver function test after 12 months of stopping the suspected drug in 21 of 66 patients followed up for more than 12 months after stopping the suspected drug AST serum aspartate aminotransferase ALT serum alanine aminotransferase ULN upper limit of normal DILI drug-induced liver injury
DISCUSSION
The spectrum of liver disease reported in our study is different from previous reports in several aspects. First, there are a large number of patients with probable ayurvedic DILI. Even if one was to use the CIOMS score of >5 for diagnosing probable ayurvedic DILI, there were 15 patients with probable ayurvedic DILI in our series. It is generally not appreciated that ayurvedic drugs may be a cause of DILI, and therefore, the history is often not volunteered by the patient and not extracted by the physician.10 Despite a systematic search for HILI in Berlin, only one case of probable HILI was identified.11
Second, 8 patients were post-liver transplant. There are few case reports of DILI after liver transplant. Drugs implicated have been azathioprine, isoniazid, sirolimus, tacrolimus, cyclosporin, amiodarone and amoxicillin–clavulanic acid.12–18 After exclusion of vascular occlusion (mainly hepatic artery thrombosis), rejection and biliary leak/stricture, ALT decrease of >50% within 8 days after withdrawal of the drug is the mainstay of diagnosis, as in our patients. Fluconazole was the most common cause of post-liver transplant DILI in our series. The incidence of hepatic injury due to antifungal drugs was 29% in a series from China.19
Third, chronic DILI (abnormal LFT after 12 months of stopping the suspected drug) was found in 2/66 patients who had a follow-up of more than 1 year. It was found only in those patients who, at 12 months, had at least one of the LFTs more than twice the ULN. In this subgroup of patients, chronic DILI occurred in 2/21 (10%). However, it is important to note that most patients with abnormal liver function more than 12 months after stopping treatment with the suspected drug do not have chronic DILI; therefore, other causes should be carefully excluded. Furthermore, 4 patients had abnormal liver function due to a second episode of DILI occurring between 6 and 12 months after stopping the initially implicated drug, either due to the same drug or another drug. This history is often not volunteered by the patient and not extracted by the physician but is important to pursue.
Conclusion
We present a series of patients with DILI with features different from previous reports. It has many patients with ayurvedic DILI and status post-liver-transplant, some had a second episode of DILI which could have been mistaken for evidence of chronicity and chronic DILI was seen in 2 patients.
ACKNOWLEDGEMENT
We are thankful to Dr G. Aithal for his suggestions.
Conflicts of interest
None declared
References
- Drug induced liver injury at a tertiary hospital in India: Etiology, clinical features and predictors of mortality. Ann Hepatol. 2017;16:442-50.
- [CrossRef] [PubMed] [Google Scholar]
- Antituberculosis therapy-induced acute liver failure: Magnitude, profile, prognosis, and predictors of outcome. Hepatology. 2010;51:1665-74.
- [CrossRef] [PubMed] [Google Scholar]
- RUCAM in drug and herb induced liver injury: The update. Int J Mol Sci. 2015;17:14.
- [CrossRef] [PubMed] [Google Scholar]
- Case definition and phenotype standardization in drug-induced liver injury. Clin Pharmacol Ther. 2011;89:806-15.
- [CrossRef] [PubMed] [Google Scholar]
- Roussel UCLAF causality assessment method for drug-induced liver injury: Present and future. Front Pharmacol. 2019;10:853.
- [CrossRef] [PubMed] [Google Scholar]
- Herbal hepatotoxicity: A tabular compilation of reported cases. Liver Int. 2012;32:1543-56.
- [CrossRef] [PubMed] [Google Scholar]
- Causality assessment in hepatotoxicity by drugs and dietary supplements. Br J Clin Pharmacol. 2008;66:758-66.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic liver injury induced by drugs: A systematic review. Liver Int. 2015;35:2343-53.
- [CrossRef] [PubMed] [Google Scholar]
- Definition and risk factors for chronicity following acute idiosyncratic drug-induced liver injury. J Hepatol. 2016;65:532-42.
- [CrossRef] [PubMed] [Google Scholar]
- Review article: Herbal and dietary supplement hepatotoxicity. Aliment Pharmacol Ther. 2013;37:3-17.
- [CrossRef] [PubMed] [Google Scholar]
- Herb-induced liver injury in the Berlin case-control surveillance study. Int J Mol Sci. 2016;17:1.
- [CrossRef] [PubMed] [Google Scholar]
- Amoxicillin/clavulanic acid-induced cholestatic liver injury after pediatric liver transplantation. Ann Transplant. 2012;17:128-31.
- [CrossRef] [PubMed] [Google Scholar]
- Acute amiodarone hepatotoxicity after liver transplantation. Transplantation. 2011;91:e62-4.
- [CrossRef] [PubMed] [Google Scholar]
- Hepatotoxicity caused by both tacrolimus and cyclosporine after living donor liver transplantation. J Nippon Med Sch. 2008;75:187-91.
- [CrossRef] [PubMed] [Google Scholar]
- Tacrolimus-induced cholestatic syndrome following pediatric liver transplantation and steroid-resistant graft rejection. Pediatr Transplant. 2006;10:220-4.
- [CrossRef] [PubMed] [Google Scholar]
- Sirolimus-associated hepatotoxicity in liver transplantation. Ann Pharmacother. 2004;38:1593-6.
- [CrossRef] [PubMed] [Google Scholar]
- Isoniazid hepatotoxicity after orthotopic liver transplantation. Mt Sinai J Med. 1996;63:364-9.
- [Google Scholar]
- Azathioprine hepatotoxicity after liver transplantation. Hepatology. 1991;14:806-10.
- [CrossRef] [PubMed] [Google Scholar]
- A clinical-pathological analysis of drug-induced hepatic injury after liver transplantation. Transplant Proc. 2007;39:3287-91.
- [CrossRef] [PubMed] [Google Scholar]




