Translate this page into:
Terbinafine-induced liver injury
Corresponding Author:
Priyanka Tolani
Department of Medicine, Northern Railway Central Hospital, Basant Lane, New Delhi
India
priyankartolani@gmail.com
| How to cite this article: Kaushal M, Tolani P, Kumar N, Sharma S. Terbinafine-induced liver injury. Natl Med J India 2017;30:321-323 |
Abstract
Terbinafine is a common antifungal agent with few reports of liver injury. We present a 64-year-old man who developed terbinafine-induced liver injury. Drug-induced liver injury is an important cause of morbidity and an early diagnosis may prevent progression to severe and chronic forms of liver injury including fulminant hepatic failure.
Introduction
Terbinafine is an antifungal agent commonly used to treat dermatophytosis. Its common adverse effects are nausea and headache. Oral terbinafine is rarely associated with the development of symptomatic hepatobiliary dysfunction. Clinicians should be aware of this rare side-effect because the liver injury may be reversible if the drug is discontinued early. We report a patient who presented to us with jaundice and was on terbinafine treatment for tinea cruris and corporis infection.
The Case
A 64-year-old man, resident of Delhi, presented to our outpatient department with complaints of yellowish discolouration of his urine and eyes for 5–6 days, clay-coloured stools for 3 days and low-grade fever for 2 days. He was known to have hypertension for the past 11 years and had been prescribed amlodipine 5 mg and atenolol 50 mg once a day. He did not drink or smoke. He was on oral terbinafine 250 mg once a day for the past 20 days for tinea cruris and corporis infection. On examination, he had icterus. Red-coloured circular lesions with central clearing were present predominantly over the chest and abdomen. These lesions had decreased in number after treatment with terbinafine. Rest of the general and systemic examination was unremarkable [Figure - 1]. On a strong suspicion of drug-induced liver injury, terbinafine was stopped.
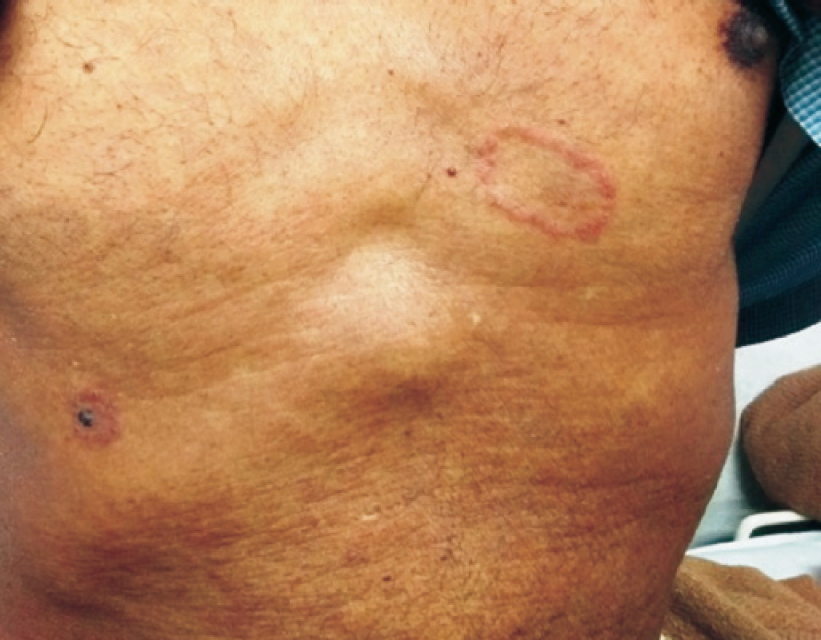 |
| Figure 1: Red-coloured itchy circular lesions with central clearing suggesting a tinea infection |
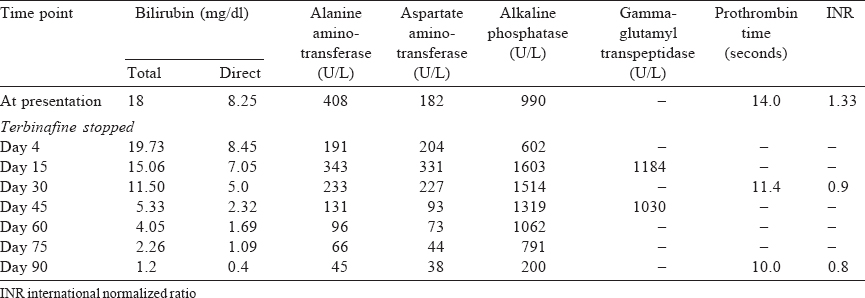
Laboratory investigations revealed deranged liver functions with a total bilirubin 18 mg/dl, direct bilirubin 8.25 mg/dl, aspartate aminotransferase 182 U/L, alanine aminotransferase 408 U/L, alkaline phosphatase (ALP) 990 U/L, total protein 5.2 g/dl, A:G ratio of 1.1, total cholesterol 403.9 mg/dl, triglycerides 295.9 mg/dl, high-density lipoprotein 9.8 mg/dl, low-density lipoprotein 377.6 mg/dl, prothrombin time 14 seconds, international normalized ratio 1.33. The haemoglobin, complete blood count and kidney function tests were normal. Immunoglobulin M (IgM) antihepatitis A virus, IgM antihepatitis E virus, hepatitis B surface antigen, hepatitis B virus DNA, hepatitis C virus (HCV) RNA and anti-HCV were negative. Autoimmune work-up (anti-saccharomyces cerevisiae antibodies, antineutrophil cytoplasmic antibodies, anti-liver/kidney microsomal-1 antibodies) was negative. Serum ceruloplasmin and glucose-6-phosphate dehydrogenase were normal. The Coombs test was also negative.
Ultrasound abdomen revealed a normal liver echotexture, intrahepatic biliary radicals, gallbladder, portal vein, spleen and no ascites. Contrast-enhanced computed tomography of the abdomen showed a partially distended gallbladder with peri-gallbladder inflammatory changes, mild thickening of the rectum with inflammatory changes in the perirectal fat suggestive of proctitis and few subcentimetric lymph nodes in the retroperi-toneum. Magnetic resonance cholangiopancreatography and sigmoidoscopy were normal. A biopsy from the rectosigmoid region showed mild non-specific inflammation. Endoscopic ultrasound-guided fine-needle aspiration cytology of the retroperitoneal lymph nodes was normal. Liver biopsy showed mild lymphocytic infiltrate in the portal areas and sinusoids. Canalicular cholestasis was evident. No changes of autoimmune hepatitis were present. It was suggestive of drug-induced cholestasis [Figure - 2].
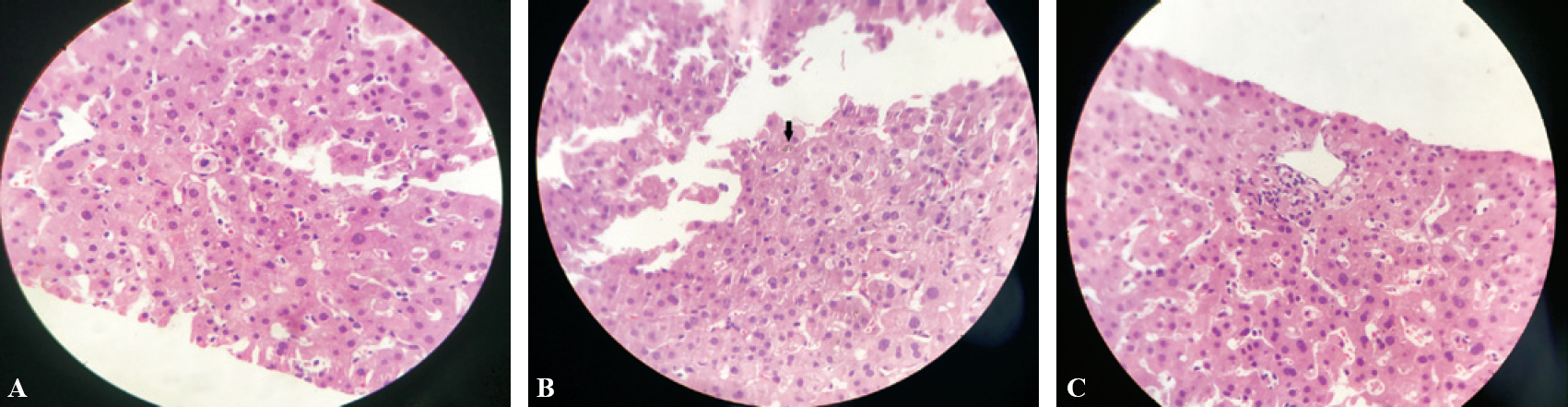 |
| Figure 2: Histopathology of the liver. A: intracellular cholestasis; B: canalicular cholestasis; C: mild lymphocytic infiltration |
The patient responded well to ursodeoxycholic acid, s-adenosylmethionine, proton pump inhibitors and intravenous fluids. His general condition and liver function tests improved. He was discharged and regularly followed up as an outpatient with liver function tests, which gradually normalized over 3 months [Table - 1].
Discussion
Terbinafine, a commonly used antifungal agent, is a synthetic allylamine available both as oral and topical formulation used primarily for dermatophytoses, especially onychomycosis. Adverse effects are rare, primarily constituting of gastrointestinal upset and headache.[1] Shortly after its introduction for medical use, drug-induced liver injury (DILI) due to terbinafine was identified. However, clinically apparent liver injury from terbinafine occurs rarely.
Terbinafine-induced liver injury is usually of cholestatic or mixed variety, injury being linked to an allylic aldehyde metabolite (7,7-dimethlyl hept-2-ene-4-ynal). Hence, it is recommended to monitor patients clinically and by measuring liver biochemistry through periodic blood tests after confirming normal liver function at the onset of therapy with terbinafine.[2]
Drug-induced liver injury is a diagnosis of exclusion. To label the liver injury as caused by a drug, a detailed history of the symptoms, time latency, use of other known hepatotoxic drugs, herbal products, alcohol and any dietary supplements is required, as well as a thorough examination and relevant investigations.[3]
The initial laboratory testing should include liver function tests and eosinophil count. To classify the pattern of injury, the R value needs to be calculated as follows: (patient's alanine amino-transferase/upper limit of normal [ULN]) / (patient's ALP/ULN). If the R value >5 the injury is of hepatocellular variety, if R <2 the injury is of cholestatic pattern, and if 2<R<5 the injury is of mixed type.[3]
In patients with hepatocellular pattern of liver injury, alternative causes such as viral hepatitis, autoimmune hepatitis, Budd-Chiari syndrome and Wilson disease have to be excluded. In the cholestatic variety, imaging studies have to be done along with serological testing.[3] The American College of Gastro-enterology recommends liver biopsy when either autoimmune hepatitis is suspected or if liver enzymes remain elevated for more than 6 months or if liver enzymes continue to rise even after stopping the offending drug.[3]
A few scales have been developed to improve the reliability of causality assessment in cases of hepatotoxicity which include the Roussel Uclaf Causality Assessment Method (RUCAM), the Council for International Organizations of Medical Sciences scale and the Maria and Victorino scale (M and V scale).[4],[5],[6]
The R value in our patient was 2.45, which was suggestive of mixed type of liver injury. The causality assessment score on the basis of RUCAM criteria was 8 and according to M and V scale was 15. Hence, an initial diagnosis of terbinafine-induced liver injury was made.
New biomarkers are being investigated as potential markers of liver injury such as microRNA-122, high-mobility group box 1, cytokeratin 18 full length and macrophage colony-stimulating factor receptor-1.[7]
Genome-wide association studies also have unearthed a number of new associations between human leucocyte antigen class I and II alleles and DILI.[7]
Finally, the treatment of DILI is withdrawal of the offending agent. Early withdrawal prevents progression to acute liver failure, but there is little firm evidence to support this. However, in some instances, a drug taken only for 2–3 days may also lead to a fatal outcome. Antihistamines such as diphenhydramine, hydroxyzine, ursodeoxycholic acid and corticosteroids may be used for symptomatic improvement in severe cases of DILI. The evidence supporting the role of any of these agents is limited.[8]
Re-exposure to a drug likely to have caused DILI is strongly discouraged, especially if the liver injury was associated with significantly raised liver enzymes (Hy law). An exception to this recommendation is in patients with life-threatening situations where a suitable alternative drug is not available.[8]
Extracorporeal systems have progressed through advances in genetically produced cell lines, stem cell-derived functional hepatocytes and various other techniques and methods of preserving hepatocytes. Advances in bioartificial livers provide the potential for benefit to patients with DILI.[8]
Last but not the least, liver transplantation provides a rescue for patients when signs of spontaneous recovery are not evident. The King's college criteria and model for end-stage liver disease score may help predict the need for liver transplantation.[8]
Conclusion
DILI is an important cause of liver injury. Both early withdrawal of the implicated drug and monitoring of liver function tests are recommended while prescribing drugs with known intrinsic or idiosyncratic hepatotoxicity.
Conflicts of interest: None
| 1. | Sheppard D, Lampiris HW. Antifungal agents. In: Katzung BG, Masters SB, Trevor AJ (eds). Basic and clinical pharmacology. 11th ed. San Francisco:The McGraw-Hill Companies; 2009:842-3. [Google Scholar] |
| 2. | Ajit C, Suvannasankha A, Zaeri N, Munoz SJ. Terbinafine-associated hepatotoxicity. Am J Med Sci 2003;325:292-5. [Google Scholar] |
| 3. | Mohankumar N, Ranjan P, Kumari A. Drug-induced liver injury: Diagnosing (and treating) it early. J Fam Pract 2015;64:634-44. [Google Scholar] |
| 4. | Fontana RJ, Seeff LB, Andrade RJ, Björnsson E, Day CP, Serrano J, et al. Standardization of nomenclature and causality assessment in drug-induced liver injury: Summary of a clinical research workshop. Hepatology 2010;52:730-42. [Google Scholar] |
| 5. | Maria VA, Victorino RM. Development and validation of a clinical scale for the diagnosis of drug-induced hepatitis. Hepatology 1997;26:664-9. [Google Scholar] |
| 6. | Lucena MI, Camargo R, Andrade RJ, Perez-Sanchez CJ, Sanchez De La Cuesta F. Comparison of two clinical scales for causality assessment in hepatotoxicity. Hepatology 2001;33:123-30. [Google Scholar] |
| 7. | Kullak-Ublick GA, Andrade RJ, Merz M, End P, Benesic A, Gerbes AL, et al Drug-induced liver injury: Recent advances in diagnosis and risk assessment. Gut 2017;66:1154-64. [Google Scholar] |
| 8. | Giordano C, Rivas J, Zervos X. An update on treatment of drug-induced liver injury. J Clin Transl Hepatol 2014;2:74-9. [Google Scholar] |
Fulltext Views
2,698
PDF downloads
523




