Translate this page into:
Balanced crystalloids in the acutely ill patient
2 Department of Critical Care Medicine, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, Uttar Pradesh, India
Corresponding Author:
Banani Poddar
Department of Critical Care Medicine, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, Uttar Pradesh
India
bananip@sgpgi.ac.in
| How to cite this article: Samal S, Mishra SB, Poddar B. Balanced crystalloids in the acutely ill patient. Natl Med J India 2019;32:218-229 |
Abstract
Administration of intravenous fluids is the most common therapy given to patients admitted to a hospital. Evidence suggests that the use of normal saline (NS) in large quantities is not without adverse effects. Balanced salt solutions (BSS) contain bicarbonate or one of its precursors that act as a buffer, and the electrolyte composition resembles that of plasma. We reviewed studies across different setups such as intensive care units (ICUs), major surgeries, renal transplants and emergency departments to identify the effect(s) of NS and to find evidence favouring the use of BSS over NS. The use of NS is strongly associated with hyperchloraemic acidosis in almost all the studies. In the largest and latest trial in ICUs, it was found that higher chloride levels were associated with renal injury. No significant difference was found in mortality in any of the trials. In surgical patients, studies found only transient hyperchloraemia and increase in the base deficit in patients receiving NS. Systematic reviews and meta-analyses did not find any significant differences in adverse outcomes such as the need for renal replacement therapy or mortality with the use of saline; however, blood chloride levels were consistently higher with saline compared to BSS. There is a need for larger trials with better methodology to determine if the physiological benefits of BSS translate into better clinical outcomes.Introduction
Almost every patient admitted to a hospital is administered intravenous (i.v.) fluids in some form for one reason or another. Fluids may be given as maintenance therapy, for replacing losses, for patients unable to take adequate fluids orally, for patients with sepsis, during or after surgery, for maintenance of organ perfusion or as a vehicle for drugs.[1] Different fluids have been formulated and tried for these purposes. Starting from normal saline (NS) to Ringer lactate (RL) and balanced salt solutions (BSS); and among colloids, from albumin to different colloids, i.v. fluids have evolved a lot.
Evolution of Intravenous Fluids
Historically, this therapy dates back to the cholera outbreak in London in 1831–32. Dr Latta first tried injecting warm water and salts into the large intestine along with intermittent oral feeds. However, he found that there was increased vomiting and purging. He, thus, thought of directly pushing fluid into the circulation. The injected fluid was made of 2–3 drachms of muriate of soda and two scruples of subcarbonate of soda in six pints of water. He injected ounce after ounce of the fluid in an old woman who showed excellent recovery from symptoms.[2],[3],[4]
Since then, for over 100 years, i.v. fluids have been an important pillar of treatment in different clinical scenarios. The first fluid to be used was ‘normal’ saline which is now considered not-so-normal as it is not physiological and is associated with side-effects such as hyperchloraemic metabolic acidosis.
Within half a century of the introduction of i.v. fluids, the next step was lactated fluids (RL/Hartmann solution) which had a bicarbonate precursor. Albumin was used intravenously in 1834. Several colloids were introduced but were rejected due to higher mortality associated with them. Widespread use of i.v. fluids, however, started only after 1950, when Dr David Massa introduced the Rochester needle.[5]
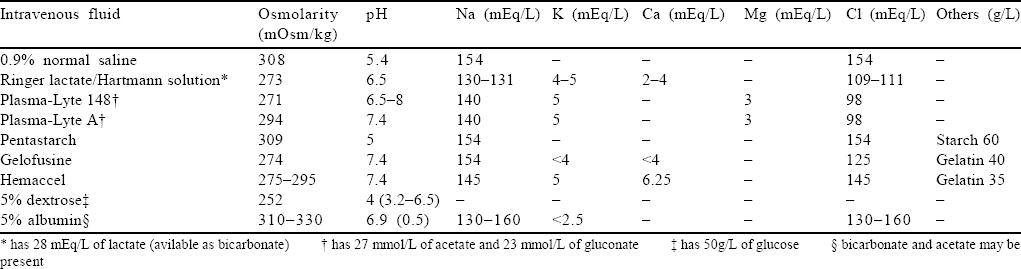
Over the years, it was found that though NS is the most commonly used fluid, its use led to the development of hyperchloraemic acidosis and increased incidence of acute kidney injury (AKI). Thus, there was a shift towards more physiological solution or BSS that would interfere less with the internal milieu and would be easier on the kidneys with lesser biochemical and physical changes .[6],[7] The newer BSS have bicarbonate or one of the precursors of bicarbonate (malate, lactate, acetate and gluconate) that acts as a buffer and also potassium, calcium and magnesium that nears the composition of plasma [Table - 1]. As of now, there is no ideal fluid to resuscitate a patient. Each has its advantages and a set of drawbacks. The choice of fluid depends on the clinical condition of the patient and, to a large extent, the physician’s choice, in keeping with the locally prevalent practices.
Evidence Related to the Use of Different Intravenous Fluids
Several studies have been done, and there are many ongoing trials to study the effect of fluids on the acid–base status and renal function, and indirectly on morbidity and mortality of the patients. We review the available studies on this topic.
One of the first studies on humans by Chowdhury et al.[8] compared the effects of 2 L of NS with Plasma-Lyte 148, infused over 1 hour each in 12 healthy volunteers in a randomized, controlled, double-blind crossover study. They found sustained hyperchloraemia with NS infusions (p<0.0001) along with a fall in strong ion deficit (SID; a measure of metabolic acidosis; p=0.025). Magnetic resonance imaging showed reduction in mean renal artery flow velocity along with reduced renal cortical tissue perfusion in volunteers who received NS infusion. No difference was noted, however, in urinary neutrophil gelatinase-associated lipocalin levels. Expansion of the intravascular space was comparable with both fluids while the expansion of the extracellular fluid compartment was more with 0.9% saline. This implied that saline may be more likely to result in interstitial oedema.[8]
The above study confirmed in humans what had been studied in animals thus far. Elevated extracellular chloride seems to have detrimental effects on vascular resistance, glomerular filtration rate (GFR) and renin activity. The postulated hypothesis is that hyperchloraemia inhibits proximal tubular chloride reabsorption, thus increasing the chloride delivery to the distal nephron. This, in turn, through negative feedback to the afferent renal vessels, decreases renal blood flow. Furthermore, stimulation of the macula densa cells due to elevated tubular chloride concentration, leads to increased afferent arteriolar resistance and decrease in GFR.[9]
Experimental studies apart, several studies in different clinical settings have been conducted, observational and otherwise. The important studies in different settings (intensive care unit [ICU], emergency department [ED], etc.) are summarized below.
Studies in the ICU [Table - 2]

Yunos et al.,[10] in a prospective, open-label, sequential-period pilot study, studied the association of AKI with administration of chloride liberal fluids (0.9% saline, 4% succinylated gelatin solution or 4% albumin solution) and chloride restrictive fluids (Hartmann solution, Plasma-Lyte 148 and chloride-poor 20% albumin) in critically ill patients. The chloride-restrictive strategy was associated with a significantly lower increase in serum creatinine levels during stay in the ICU. The chloride restrictive group had lower incidence of injury and failure class of risk, injury, failure, loss of kidney function and end-stage kidney disease classification defined AKI and reduced use of renal replacement therapy (RRT; p=0.05). No differences were found in ICU mortality (p=0.42), in-hospital mortality (p=0.44), length of stay in ICU (p=0.52) or long-term dialysis requirements (p=0.95).[10] They extended their original study by 12 months (additional 6 months before and after the change of fluid policy) and found similar results.[11]
Three retrospective studies subsequently found similar results regarding the association of mortality with serum chloride levels. Raghunathan et al.[12] (53 448 patients), Shaw et al.[13] (109 836 patients) and Zampieri et al.[14] (10 249 patients) in their respective studies found a significantly higher rate of mortality in patients with higher levels or with greater rise in serum chloride levels (who either received chloride-rich fluids or NS compared to BSS or RL solution).[12],[13],[14]
One of the few studies that did not find any change in the metabolic milieu or outcome was done by Young et al. as a double-blind, cluster randomized, double crossover trial in mixed ICUs (medical, surgical, cardiothoracic and vascular surgical)— the SPLIT randomized clinical trial, to determine the effect of buffered crystalloid compared with saline on renal complications. Crossovers occurred so that each ICU used each fluid twice over the 28 weeks of the study; 1152 of 1162 patients (99.1%) receiving buffered crystalloid and 1110 of 1116 patients (99.5%) receiving saline were analysed. They did not find any significant difference in the incidence of AKI (doubling of creatinine or increase by 0.5 mg/dl with levels ≥3.96 mg/dl), use of RRT or hospital mortality (p=0.77, 0.91, 0.40, respectively).[15] Unfortunately, this trial had several limitations. The sample size was not calculated and hence, the study was inadequately powered to detect relatively small, though potentially important, differences in the risk of toxicity between fluid types. The study cohort did not have a large number of patients at increased risk for adverse kidney outcomes. The timing and frequency of serum creatinine measurements were not standardized.
Similarly, two other studies did not find adverse outcomes associated with saline administration. Verma et al. conducted a multicentre, double-blind, randomized controlled trial (pilot study) in adult patients who were prescribed crystalloids for resuscitation in ICUs. They studied the difference between NS and Plasma- Lyte 148 administration for up to 4 days after admission to ICU. They found no significant difference between the groups in median base excess (p=0.42), incidence of AKI (p=0.48), peak creatinine levels (p=0.92) and hospital mortality. However, there was significant hyperchloraemia (p=0.01) in the NS group.[16] Semler et al. did a cluster randomized, multiple crossover trial of 974 adults to compare saline and balanced crystalloids. Saline (0.9% sodium chloride) and balanced crystalloids (lactated Ringer solution or Plasma-Lyte A) were used alternately on a monthly basis. The parameters studied were major adverse kidney events within 30 days (MAKE30), a composite of death, dialysis or persistent renal dysfunction in relation to the amount of fluid received by the patient. There were no statistically significant differences observed in MAKE30 between the groups (24.7% v. 24.6%; p=0.98).[17]
The largest and latest trial was conducted by Semler et al.,[18] a pragmatic, cluster randomized, multiple crossover trial in five ICUs, where 15 802 adults were randomized to receive either saline (0.9% NaCl) or balanced crystalloids (RL or Plasma-Lyte A)—the SMART trial. MAKE30, a composite of death from any cause, new RRT, or persistent renal dysfunction (defined as an elevation of the creatinine level to ≥200% of baseline) were studied at hospital discharge or 30 days, whichever occurred first. It was found that 1139 (14.3%) of 7942 patients receiving balanced crystalloid had a MAKE30, whereas 1211 of 7860 patients (15.4%) in the saline group had the same and this difference was statistically significant (p=0.04). The incidence of hospital mortality at 30 days (p=0.06), new RRT (p=0.08) and persistent renal dysfunction (p=0.60) were lower in the balanced crystalloid group, though not significant.[18]
In a meta-analysis of randomized controlled trials comparing balanced crystalloids and isotonic saline by Zayed et al., primary outcome measures of either hospital mortality or incidence of AKI or both, at the longest follow-up period, were considered.[19] Only prospective randomized controlled trials in an ICU were considered (>19 000 patients from six trials). They did not find any significant difference in the incidence of hospital mortality (11.5% v. 12.2%, OR 0.92; 95% CI 0.85–1.01; p=0.09; I2=0%) or AKI (12% v. 12.7%, OR 0.92; 95% CI 0.84–1.01; p=0.1; I2=0%) Moreover, they did not find any significant difference in overall ICU mortality or the need of new RRT in critically ill patients. One of the limitations of this meta-analysis was that it included a single large trial.
Studies in Renal Transplant Recipients [Table - 3]
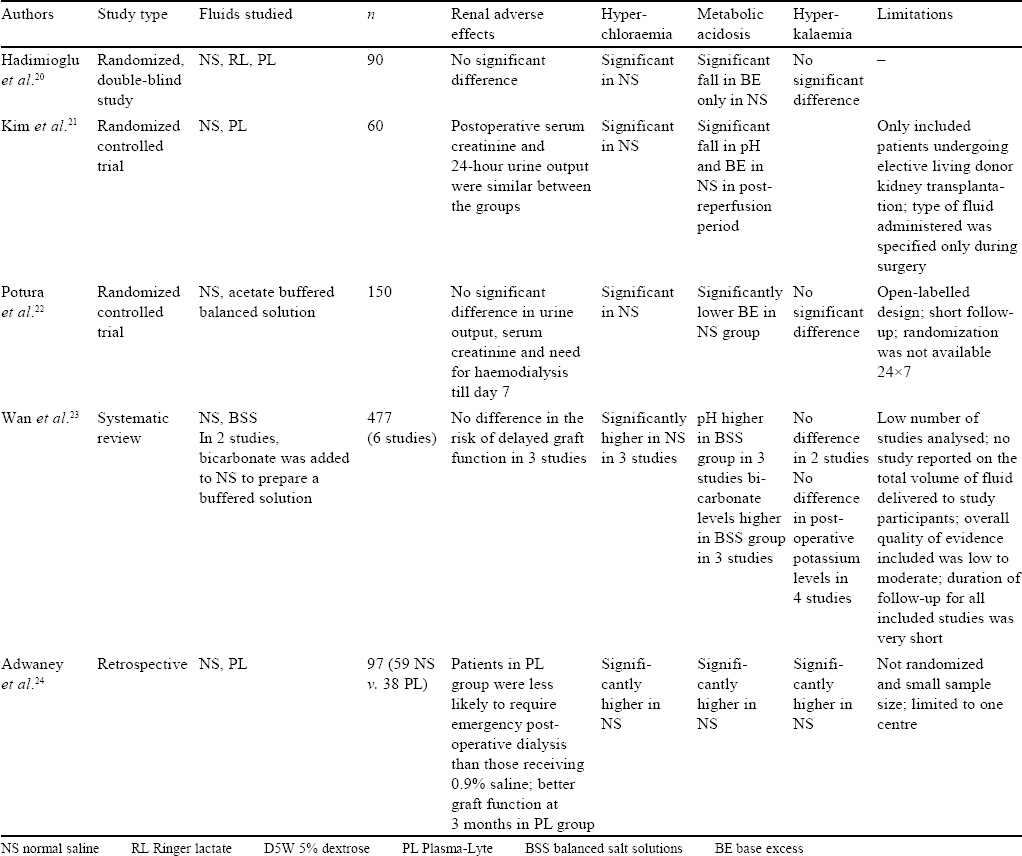
Patients undergoing renal transplantation are administered large volumes of crystalloid in the perioperative period, and the choice of fluid could potentially influence acid–base balance, electrolyte concentration (specifically the incidence of hyperkalaemia) and graft function.
Three randomized trials regarding the choice of fluids administered either during live or cadaveric renal transplant found that balanced solutions only provided a better acid–base balance or biochemical profile in patients with no significant differences in the incidence of reduced urine output, renal failure, need for dialysis, mortality or graft rejection. All the three trials deemed NS to be equally safe for use in patients undergoing renal transplant.[20],[21],[22]
Wan et al., in a Cochrane review of six studies comprising 477 participants, reviewed the use of different i.v. fluids in renal transplant patients. Incidence of hyperchloraemic acidosis was higher in patients who received NS in the perioperative period.[23]
Contrary to the above studies, a retrospective study by Adwaney et al. of 97 patients who had undergone renal transplant showed that patients receiving Plasma-Lyte were less likely to need emergency RRT (OR 0.15; p=0.004) compared to those receiving NS. Patients receiving Plasma-Lyte had a lower incidence of hyperkalaemia, acidosis, length of stay and better graft function at 3 months and better diuresis than those who received NS.[24]
Studies in Patients Undergoing Major Abdominal Surgeries [Table - 4]
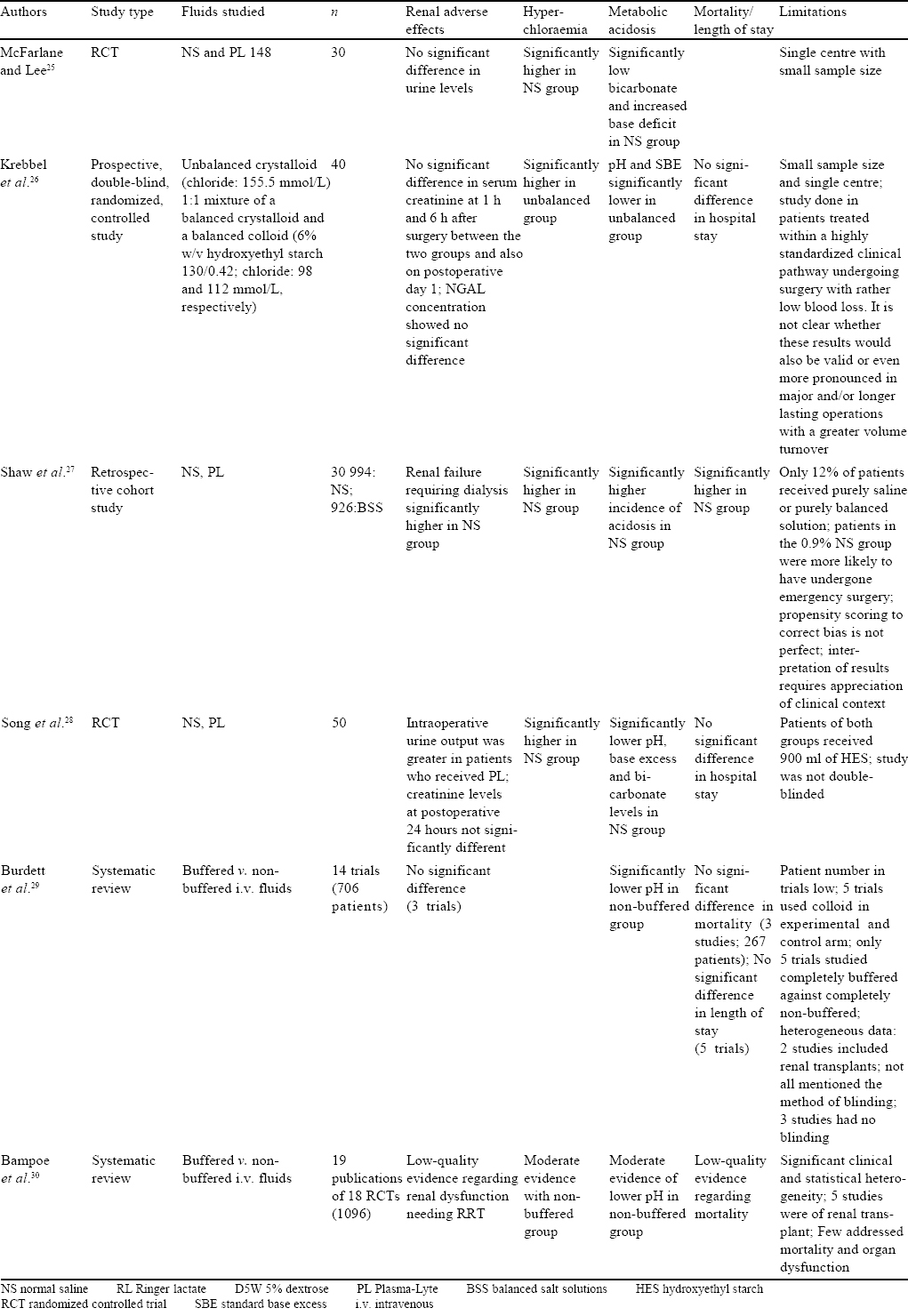
Early data on the effects of infusing NS came from the operating room. In an early study conducted by McFarlane and Lee,[25] 30 patients undergoing hepatobiliary or pancreatic surgeries were randomized to receive saline or Plasma-Lyte 148. Patients administered saline had significantly higher chloride levels, lower standard bicarbonate and higher base deficit. The authors opined that exclusive administration of saline could give a temporary hyperchloraemic acidosis which could be interpreted as being pathological. Similar results were seen in 40 patients randomized to receive a 1:1 mixture of balanced crystalloids and balanced colloids and compared with unbalanced crystalloid (buffer-free Ringer solution containing chloride 155.5 mEq/L) for goal-directed therapy during elective hip replacement surgery.[26]
In a retrospective cohort study of a large American database, Shaw et al. studied over 30 000 patients who received i.v. fluids for major open abdominal surgeries.[27] They found significantly lower incidence of hospital mortality in patients who received balanced solutions (2.9%) in comparison to patients who received NS (5.6%, p<0.001). Further, receipt of balanced fluids was associated with a lower incidence of complications (OR 0.79, 95% CI 0.66–0.97), specifically lower postoperative infections (p=0.006), lower incidence of renal failure requiring dialysis (p<0.001), blood transfusions (p<0.001), electrolyte disturbances (p=0.046) and acid–base abnormalities (p=0.02).
Song et al. randomized 50 patients undergoing spinal fusion surgery to receive either 0.9% saline or Plasma-Lyte in the perioperative period. The outcomes measured included coagulation abnormalities, intraoperative blood loss, acid–base status, electrolytes and renal functions. In contrast to Plasma-Lyte, fluid therapy with 0.9% saline resulted in transient hyperchloraemic acidosis, while coagulation tests and the amount of blood loss were similar in the groups.[28]
There are two Cochrane systematic reviews regarding the use of i.v. fluids in the perioperative period; one by Burdett et al. published in 2012 and the other by Bampoe et al. in 2017.[29],[30] Randomized trials of buffered versus non-buffered fluids given in the perioperative period were included, with 706 patients in the first review and 1096 patients in the second. No significant difference was found with respect to mortality and postoperative need of RRT with either fluid. The only difference between the groups was a significantly higher incidence of hyperchloraemic acidosis in patients who received NS. However, this difference was corrected by postoperative day 1. In addition, Burdett et al. did not find any difference in intraoperative blood loss or transfusion requirement and length of hospital stay.
Studies in Trauma and Emergency [Table - 5]
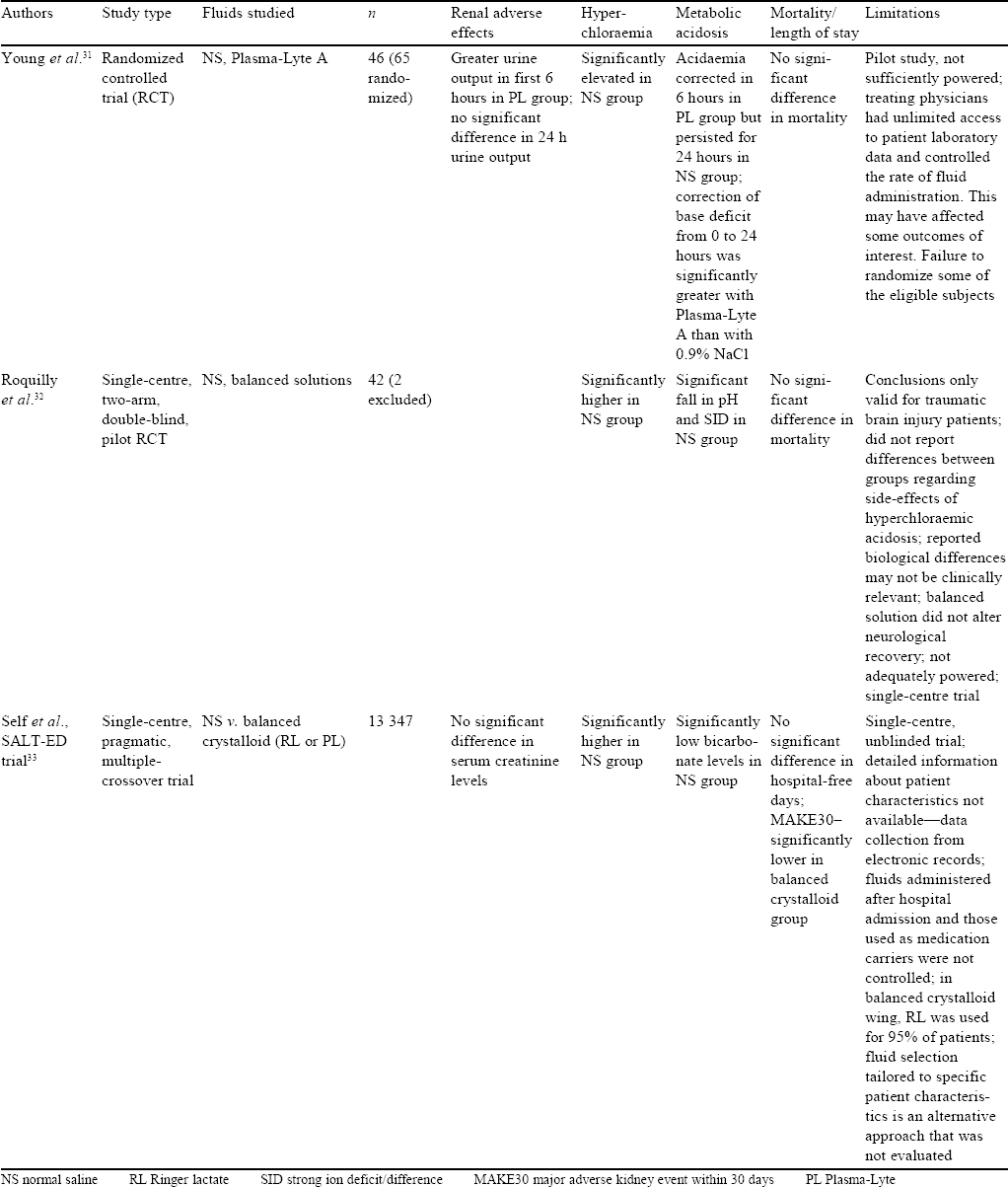
Among other patients who require large volumes of resuscitation fluids are those with trauma and/or presenting to the ED. Young et al. randomized 65 adult trauma patients requiring blood transfusion, tracheal intubation or surgery within 60 minutes of arrival to their centre to receive either saline or Plasma-Lyte A for resuscitation in the first 24 hours after injury. They hypothesized that Plasma-Lyte A would better correct the base deficit 24 hours after injury. The mean improvement in base excess from 0 to 24 hours was significantly greater with Plasma-Lyte A than with NS, arterial pH was greater and serum chloride was lower with Plasma-Lyte A than with saline. However, there was no significant difference with respect to mortality, volumes of study fluid administered and 24-hour urine output.[31]
In a randomized double-blind pilot study, Roquilly et al. compared saline to balanced solution in 42 severely brain-injured patients (who received the fluid for 48 hours).[32] There was a higher incidence of hyperchloraemic acidosis and lower SID within 48 hours in the patients who received NS in comparison to those who received balanced solution. Importantly, intracranial pressure was not different between the two groups, though this study was not powered for this endpoint.
Self et al. compared BSS (RL and Plasma-Lyte A) with NS in a single-centre, pragmatic, multiple crossover trial of saline against RL or Plasma-Lyte in the ED.[33] Clinical effects of crystalloids were studied in the ED among non-critically ill patients. Patients receiving <500 ml of crystalloids were excluded. The primary outcome studied was hospital-free days to day 28, and the secondary outcomes were MAKE30, AKI of stage 2 or higher according to the Kidney disease: Improving global outcomes guidelines and hospital deaths. No difference in the groups was observed in hospital-free days (median 25 days in both groups; adjusted OR with balanced crystalloids 0.98; 95% CI 0.92–1.04; p=0.41). However, balanced crystalloids resulted in lower incidence of MAKE30 than saline (4.7% v. 5.6%; adjusted OR 0.82; 95% CI 0.70–0.95; p=0.01). The limitations of this study were that it was done at a single centre and was not blinded. Further, detailed patient information was not available and fluids given to patients after being moved from ED were not controlled.[33]
Recent Meta-Analyses
An effective method of summarizing results of individual trials comes from systematic reviews and meta-analysis. A few meta-analyses have summarized the effects of balanced fluids in comparison to saline in perioperative and ICU patients. Spurious conclusions in systematic reviews with traditional meta-analyses can be reduced using trial sequential analysis (TSA). Several empirical studies have demonstrated that the TSA provides better control of type I errors and of type II errors than the traditional naive meta-analysis.
A systematic review and meta-analysis of randomized trials comparing fluid resuscitation with balanced solutions versus isotonic saline by Serpa Neto et al. included eight trials in operation rooms and three in ICUs. The analysis of these trials involving 2703 patients did not find any significant difference in the incidence of hospital mortality, the occurrence of AKI and the need of RRT. However, significantly lower chloride levels were found in patients receiving BSS (although without any change in arterial pH).[34]
Kawano-Dourado et al.[35] did a systematic review and included trial sequential analyses to assess the risk of bias of individual trials and the overall quality of evidence. They assessed whether the use of low chloride solutions in unselected critically ill or perioperative adult patients for maintenance or resuscitation reduces mortality and RRT use when compared to high-chloride fluids. A total of 3710 patients were included in the mortality analysis and 3724 in the RRT analysis. No significant difference in mortality (OR 0.90; 95% CI 0.69–1.17; p=0.44; I2=0%) or RRT use (OR 1.12; 95% CI 0.80–1.58; p=0.52; I2=0%) was found.[35] However, the authors opined that the overall pooled sample size was small and the volume of study fluid was limited; thus, the data were underpowered to detect potentially important differences.
Similar results were found in a systematic review and metaanalysis with TSA of nine trials (six ICU based, two focused on acute pancreatitis and one included trauma patients) by Liu et al.[36] A total of 19 203 patients who received either balanced crystalloids (RL, Plasma-Lyte A and Plasma-Lyte 148) or NS were analysed for mortality, AKI and RRT use. However, the TSA did not provide any conclusive evidence favouring the findings.
The above findings were refuted in a meta-analysis with TSA of eight studies by Xue et al.;[37] which included 19 301 patients and compared balanced crystalloids with NS in critically ill adults. The authors showed significantly longer RRT-free days (p<0.001), less risk of increase in serum concentrations of chloride (p<0.001), less risk of decline in serum base deficit (p=0.004), longer ventilator-free days (p<0.001) and vasopressor-free days (p=0.02) in patients who received balanced crystalloids. Survival in hospital did not show any statistically significant difference (p=0.06) between the two groups. However, subgroup analysis favoured improved survival in the BSS group in septic patients (p=0.02) and non-traumatic brain injury patients (p=0.02). In this study too, TSA did not find conclusive evidence and warranted a larger sample size.
Huang et al. did a meta-analysis of nine trials (871 patients) comparing BSS and NS in non-renal surgical patients.[38] BSS provided significantly better acid–base balance and lower chloride values than NS.
Studies Among Children [Table - 6]
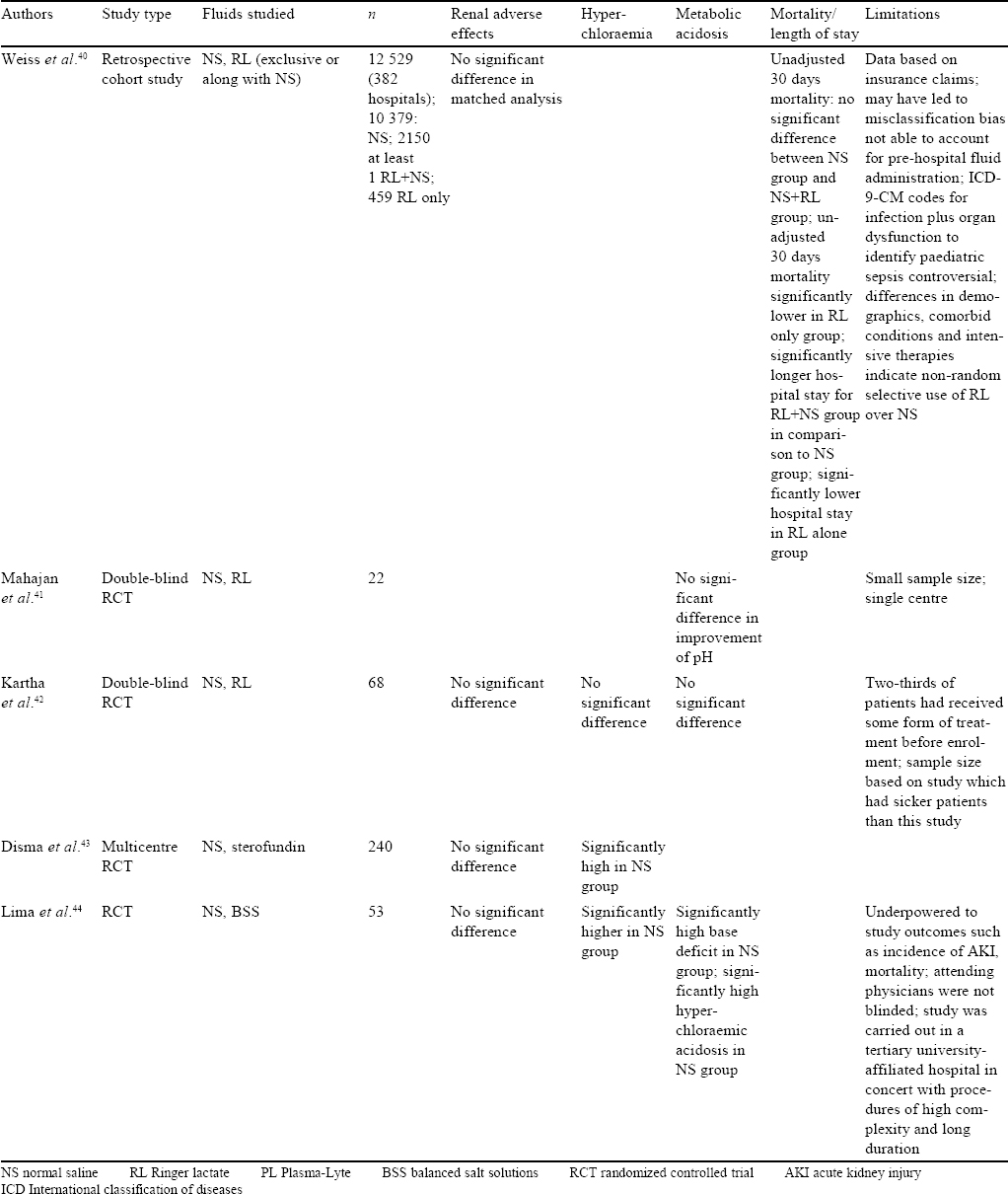
Literature is scarce regarding the choice of crystalloids for acutely ill children. Most recommendations extrapolate studies available among adults and suggest that balanced fluids may be safer for fluid resuscitation.[39]
In a matched retrospective cohort study from 382 American hospitals for children, data from 12 529 children with severe sepsis/septic shock was studied. Outcomes were compared between children who received RL during resuscitation to those who received NS. There were no differences in 30-day hospital mortality, AKI or need for new dialysis. The patients who received any amount of RL had a longer hospital stay of 2.4 days (95% CI 1.4–5 days; p<0.01).[40] This could possibly be attributed to the larger crystalloid volumes received by this group of patients.
A few small randomized trials in different settings (diarrhoeal dehydration, diabetic ketoacidosis) showed mixed results. However, it is important to note that these studies compared RL to NS. Twenty-two children with acute diarrhoeal, severe dehydration were randomized to receive either RL or NS for fluid resuscitation. There was no difference in improvement in pH over the baseline between RL and NS, though the volume of fluid required in the RL group was lesser.[41] In a similar study, among 68 children with acute diarrhoea and severe dehydration, resuscitation with RL or NS was associated with similar clinical improvement and resolution of pH. No significant differences were observed in secondary outcomes in electrolytes, renal and blood gas parameters, median time to start oral feeding and hospital stay.[42]
In a randomized controlled open trial, 240 children between 1 and 36 months undergoing major surgery were assessed for changes in plasma chloride concentrations using either NS or a balanced crystalloid solution (sterofundin) intraoperatively. The mean change in chloride levels was greater in the NS group (median 4; interquartile range [IQR] 2, 6) compared to the sterofundin group (median 2; IQR 1, 49; p=0.0001). Changes in serum magnesium were also lesser in the sterofundin group (p<0.001). No other significant electrolyte differences or change in urea/creatinine levels were seen.[43]
In another trial in children undergoing resection of brain tumours, 53 children between 6 months to 12 years were randomized to receive either NS or Plasma-Lyte A during and for 24 hours after surgery. Children in the saline group had an increase in serum chloride and base excess postoperatively, but this hyperchloraemic metabolic acidosis resolved by day 1 postoperatively. There was no difference in brain oedema (as assessed by the neurosurgeons). There was no significant renal dysfunction noted.[44]
On-Going and New Trials
The BaSICS trial by Zampieri et al. aims to provide an answer to whether a balanced crystalloid, compared with saline, improves 90-day all-cause mortality in critically ill patients. Further, the effect of a slow infusion rate as against a rapid infusion rate would also be tested. A total of 11 000 patients will be recruited from nearly 100 ICUs in Brazil.[45]
The PLUS trial by Hammond et al. will study the effect of Plasma-Lyte and saline on 90-day all-cause mortality and several other secondary outcomes in about 50 ICUs in Australia and New Zealand.[46]
Conclusion
Intravenous fluids are not without side-effects and judicious use is required, as with all other drugs. It is still inconclusive if the high chloride load is only because of the type of i.v. fluid. There is enough evidence that NS causes biochemical disturbances and may predispose to renal injury. Small effect sizes for biochemical outcomes and lack of correlated clinical follow-up data mean that robust conclusions on major morbidity and mortality associated with buffered versus non-buffered fluid choices are still lacking. Larger studies are needed to assess these relevant clinical outcomes.
Several aspects of i.v. fluid therapy remain unexplored. Further studies are needed to explore if the rate of fluid administration, the time and volume of fluid administered have any role in these biochemical changes, renal injury or mortality.
Hence, the results of the on-going trials are awaited for any conclusive evidence for better outcomes in administering particular types of i.v. fluids, a necessary component of patient care.
Conflicts of interest. None declared
| 1. | National Clinical Guideline Centre (UK). Intravenous fluid therapy: Intravenous fluid therapy in adults in hospital. (NICE Clinical Guidelines, No. 174). London:Royal College of Physicians (UK); 2013. Available at www.ncbi.nlm.nih.gov/books/ NBK247761/ . (accessed on 17 Mar 2019) [Google Scholar] |
| 2. | Masson AH. Latta - Pioneer in saline infusion. Br J Anaesth 1971 ;43:681-6. [Google Scholar] |
| 3. | Cosnett JE. The origins of intravenous fluid therapy. Lancet 1989;1:768-71. [Google Scholar] |
| 4. | Foex BA. How the cholera epidemic of 1831 resulted in a new technique for fluid resuscitation. Emerg Med J 2003;20:316-18. [Google Scholar] |
| 5. | Southorn PA, Narr BJ. The Massa or Rochester plastic needle. Mayo Clin Proc 2008;83:1165-7. [Google Scholar] |
| 6. | Morgan TJ, Venkatesh B. Designing ‘balanced’ crystalloids. Crit Care Resusc 2003;5:284-91. [Google Scholar] |
| 7. | Lobo DN. Intravenous 0.9% saline and general surgical patients: A problem, not a solution. Ann Surg 2012;255:830-2. [Google Scholar] |
| 8. | Chowdhury AH, Cox EF, Francis ST, Lobo DN. A randomized, controlled, double-blind crossover study on the effects of 2-L infusions of 0.9% saline and plasma-lyte® 148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers. Ann Surg 2012;256:18-24. [Google Scholar] |
| 9. | Wilcox CS. Regulation of renal blood flow by plasma chloride. J Clin Invest 1983;71:726-35. [Google Scholar] |
| 10. | Yunos NM, Bellomo R, Hegarty C, Story D, Ho L, Bailey M. Association between a chloride-liberal vs. chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA 2012;308:1566-72. [Google Scholar] |
| 11. | Yunos NM, Bellomo R, Glassford N, Sutcliffe H, Lam Q, Bailey M. Chloride-liberal vs. chloride-restrictive intravenous fluid administration and acute kidney injury: An extended analysis. Intensive Care Med 2015;41:257-64. [Google Scholar] |
| 12. | Raghunathan K, Shaw A, Nathanson B, Stürmer T, Brookhart A, Stefan MS, et al. Association between the choice of IV crystalloid and in-hospital mortality among critically ill adults with sepsis. Crit Care Med 2014;42:1585-91. [Google Scholar] |
| 13. | Shaw AD, Raghunathan K, Peyerl FW, Munson SH, Paluszkiewicz SM, Schermer CR. Association between intravenous chloride load during resuscitation and in-hospital mortality among patients with SIRS. Intensive Care Med 2014;40: 1897-905. [Google Scholar] |
| 14. | Zampieri FG, Ranzani OT, Azevedo LC, Martins ID, Kellum JA, Libório AB. Lactated Ringer is associated with reduced mortality and less acute kidney injury in critically ill patients: A retrospective cohort analysis. Crit Care Med 2016;44: 2163-70. [Google Scholar] |
| 15. | Young P, Bailey M, Beasley R, Henderson S, Mackle D, McArthur C, et al. Effect of a buffered crystalloid solution vs. saline on acute kidney injury among patients in the intensive care unit: The SPLIT randomized clinical trial. JAMA 2015;314: 1701-10. [Google Scholar] |
| 16. | Verma B, Luethi N, Cioccari L, Lloyd-Donald P, Crisman M, Eastwood G, et al. A multicentre randomised controlled pilot study of fluid resuscitation with saline or Plasma-lyte 148 in critically ill patients. Crit Care Resusc 2016;18:205-12. [Google Scholar] |
| 17. | Semler MW, Wanderer JP, Ehrenfeld JM, Stollings JL, Self WH, Siew ED, et al Balanced crystalloids versus saline in the intensive care unit. The SALT randomized trial. Am J Respir Crit Care Med 2017;195:1362-72. [Google Scholar] |
| 18. | Semler MW, Self WH, Wanderer JP, Ehrenfeld JM, Wang L, Byrne DW, et al Balanced crystalloids versus saline in critically ill adults. N Engl J Med 2018;378: 829-39. [Google Scholar] |
| 19. | Zayed YZ, Aburahma AM, Barbarawi MO, Hamid K, Banifadel MR, Rashdan L, et al. Balanced crystalloids versus isotonic saline in critically ill patients: Systematic review and meta-analysis. J Intensive Care 2018;6:51. [Google Scholar] |
| 20. | Hadimioglu N, Saadawy I, Saglam T, Ertug Z, Dinckan A. The effect of different crystalloid solutions on acid-base balance and early kidney function after kidney transplantation. Anesth Analg 2008;107:264-9. [Google Scholar] |
| 21. | Kim SY, Huh KH, Lee JR, Kim SH, Jeong SH, Choi YS. Comparison of the effects of normal saline versus Plasmalyte on acid-base balance during living donor kidney transplantation using the Stewart and base excess methods. Transplant Proc 2013;45:2191-6. [Google Scholar] |
| 22. | Potura E, Lindner G, Biesenbach P, Funk GC, Reiterer C, Kabon B, et al. An acetate-buffered balanced crystalloid versus 0.9% saline in patients with end-stage renal disease undergoing cadaveric renal transplantation: A prospective randomized controlled trial. Anesth Analg 2015;120:123-9. [Google Scholar] |
| 23. | Wan S, Roberts MA, Mount P. Normal saline versus lower-chloride solutions for kidney transplantation. Cochrane Database Syst Rev 2016;8:CD010741. [Google Scholar] |
| 24. | Adwaney A, Randall DW, Blunden MJ, Prowle JR, Kirwan CJ. Perioperative Plasma-lyte use reduces the incidence of renal replacement therapy and hyperkalaemia following renal transplantation when compared with 0.9% saline: A retrospective cohort study. Clin Kidney J 2017;10:838^4. [Google Scholar] |
| 25. | McFarlane C, Lee A. A comparison of Plasmalyte 148 and 0.9% saline for intraoperative fluid replacement. Anaesthesia 1994;49:779-81. [Google Scholar] |
| 26. | Krebbel H, Feldheiser A, Müller O, Boemke W, Sander M, Perka C, et al Influence of goal-directed therapy with balanced crystalloid-colloid or unbalanced crystalloid solution on base excess. J Int Med Res 2014;42:468-86. [Google Scholar] |
| 27. | Shaw AD, Bagshaw SM, Goldstein SL, Scherer LA, Duan M, Schermer CR, et al. Major complications, mortality, and resource utilization after open abdominal surgery: 0.9% saline compared to Plasma-lyte. Ann Surg 2012;255:821-9. [Google Scholar] |
| 28. | Song JW, Shim JK, Kim NY, Jang J, Kwak YL. The effect of 0.9% saline versus Plasmalyte on coagulation in patients undergoing lumbar spinal surgery; a randomized controlled trial. Int J Surg 2015;20:128-34. [Google Scholar] |
| 29. | Burdett E, Dushianthan A, Bennett-Guerrero E, Cro S, Gan TJ, Grocott MP, et al. Perioperative buffered versus non-buffered fluid administration for surgery in adults. Cochrane Database Syst Rev 2012;12:CD004089. [Google Scholar] |
| 30. | Bampoe S, Odor PM, Dushianthan A, Bennett-Guerrero E, Cro S, Gan TJ, et al. Perioperative administration of buffered versus non-buffered crystalloid intravenous fluid to improve outcomes following adult surgical procedures. Cochrane Database Syst Rev 2017;9:CD004089. [Google Scholar] |
| 31. | Young JB, Utter GH, Schermer CR, Galante JM, Phan HH, Yang Y, et al. Saline versus Plasma-lyte A in initial resuscitation of trauma patients: A randomized trial. Ann Surg 2014;259:255-62. [Google Scholar] |
| 32. | Roquilly A, Loutrel O, Cinotti R, Rosenczweig E, Flet L, Mahe PJ, et al. Balanced versus chloride-rich solutions for fluid resuscitation in brain-injured patients: A randomised double-blind pilot study. Crit Care 2013;17:R77. [Google Scholar] |
| 33. | Self WH, Semler MW, Wanderer JP, Wang L, Byrne DW, Collins SP, et al. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med 2018;378:819-28. [Google Scholar] |
| 34. | Serpa Neto A, Martin Loeches I, Klanderman RB, Freitas Silva R, Gama de Abreu M, Pelosi P, et al. Balanced versus isotonic saline resuscitation—a systematic review and meta-analysis of randomized controlled trials in operation rooms and intensive care units. Ann Transl Med 2017;5:323. [Google Scholar] |
| 35. | Kawano-Dourado L, Zampieri FG, Azevedo LC, Corrêa TD, Figueiró M, Semler MW, et al. Low- versus high-chloride content intravenous solutions for critically ill and perioperative adult patients: A systematic review and meta-analysis. Anesth Analg 2018;126:513-21. [Google Scholar] |
| 36. | Liu C, Lu G, Wang D, Lei Y, Mao Z, Hu P, et al. Balanced crystalloids versus normal saline for fluid resuscitation in critically ill patients: A systematic review and meta-analysis with trial sequential analysis. Am J Emerg Med 2019. pii: S0735-6757(19)30149-4. [Google Scholar] |
| 37. | Xue M, Zhang X, Liu F, Chang W, Xie J, Xu J, et al. Effects of chloride content of intravenous crystalloid solutions in critically ill adult patients: A meta-analysis with trial sequential analysis of randomized trials. Ann Intensive Care 2019;9:30. [Google Scholar] |
| 38. | Huang L, Zhou X, Yu H. Balanced crystalloids vs. 0.9% saline for adult patients undergoing non-renal surgery: A meta-analysis. Int J Surg 2018 ;51:1-9. [Google Scholar] |
| 39. | Long E, Duke T. Fluid resuscitation therapy for paediatric sepsis. JPaediatr Child Health 2016;52:141-6. [Google Scholar] |
| 40. | Weiss SL, Keele L, Balamuth F, Vendetti N, Ross R, Fitzgerald JC, et al Crystalloid fluid choice and clinical outcomes in pediatric sepsis: A matched retrospective cohort study. J Pediatr 2017;182:304-10. [Google Scholar] |
| 41. | Mahajan V, Sajan SS, Sharma A, Kaur J. Ringers lactate vs. normal saline for children with acute diarrhea and severe dehydration—a double blind randomized controlled trial. Indian Pediatr 2012;49:963-8. [Google Scholar] |
| 42. | Kartha GB, Rameshkumar R, Mahadevan S. Randomized double-blind trial of Ringer lactate versus normal saline in pediatric acute severe diarrheal dehydration. J Pediatr Gastroenterol Nutr 2017;65:621-6. [Google Scholar] |
| 43. | Disma N, Mameli L, Pistorio A, Davidson A, Barabino P, Locatelli BG, et al. A novel balanced isotonic sodium solution vs. normal saline during major surgery in children up to 36 months: A multicenter RCT. Paediatr Anaesth 2014;24:980-6. [Google Scholar] |
| 44. | Lima MF, Neville IS, Cavalheiro S, Bourguignon DC, Pelosi P, Malbouisson LM, et al. Balanced crystalloids versus saline for perioperative intravenous fluid administration in children undergoing neurosurgery: A randomized clinical trial. J Neurosurg Anesthesiol 2019;31:30-5. [Google Scholar] |
| 45. | Zampieri FG, Azevedo LC, Corrêa TD, Falavigna M, Machado FR, Assunção MS, et al. Study protocol for the balanced solution versus saline in intensive care study (BaSICS): A factorial randomised trial. Crit Care Resusc 2017;19:175-82. [Google Scholar] |
| 46. | Hammond NE, Bellomo R, Gallagher M, Gattas D, Glass P, Mackle D, et al. The Plasma-lyte 148 v saline (PLUS) study protocol: A multicentre, randomised controlled trial of the effect of intensive care fluid therapy on mortality. Crit Care Resusc 2017;19:239-46. [Google Scholar] |
Fulltext Views
3,660
PDF downloads
1,647




