Translate this page into:
Direct ophthalmoscopy as a screening tool to study retinal vascular changes in acute mountain sickness in response to recent ascent to high altitude: A pilot study
Correspondence to SHIBU SASIDHARAN; shibusasi@gmail.com
[To cite: Gupta A, Rana V, Sasidharan S. Direct ophthalmoscopy as a screening tool to study retinal vascular changes in acute mountain sickness in response to recent ascent to high altitude: A pilot study. Natl Med J India 2023;36:358–60. DOI: 10.25259/NMJI_358_21]
Abstract
Background
Advanced diagnostics are not easily accessible in austere topographical locations. We documented retinal changes in patients with acute mountain sickness (AMS+) and compared these with asymptomatic individuals (AMS–) with recent induction into high altitude using direct ophthalmoscopy as a screening tool.
Methods
We evaluated 97 individuals (43 AMS– and 54 AMS+) who were inducted to an altitude 3800 m above sea level by direct ophthalmoscopy after pupillary dilatation, on day 2 of arrival.
Results
Retinal vein dilatation was seen in 36 (66.7%) AMS+ v. 14 (32.6%) AMS– (p<0.01), hyperaemia of the optic disc in 30 (55.6%) AMS+ v. 14 (32.6%) AMS– (p<0.05), hyperaemia of the optic disc along with retinal vein dilatation in 27 (50%) AMS+ v. 9 (20.9%) AMS– (p<0.01), retinal vein tortuosity in 12 (22.2%) AMS+ v. 3 (7%) AMS– (p<0.02). In AMS+ with retinal vein dilatation 17 (50%) had SpO2 >91% and 19 (79.2%) had SpO2 <91% (p<0.01). An AMS score of >5 was recorded in 25 (69.4%; p<0.001) with venular dilatation and in 19 (52.8%; p<0.001) who were AMS+ with an induction number ≥3 had retinal dilatation.
Conclusion
Acute hypobaric hypoxia causes retinal venous dilatation, tortuosity and hyperaemia of the optic disc in those with AMS and correlates directly with SpO2 levels. The incidence of retinal vein dilatation increases with frequent re-entry into high altitude and more severe symptoms of AMS. Hence, all those being inducted to high altitude should be screened for retinal vascular changes.
INTRODUCTION
Acute mountain sickness (AMS) is described as an altitude-related potentially life-threatening condition with headache as the main clinical symptom.1 The pathophysiology of AMS remains a conundrum. Even though several mechanisms have been described to contribute to the development of AMS, the most crucial factor is hypoxia of high altitude. Hypoxia eventually leads to some degree of cerebral oedema, which has been seen in computerized tomography (CT) and magnetic resonance imaging (MRI) scans after 48 hours stay at 3700 m.1 However, these advanced diagnostic facilities are not easily accessible at high altitudes. Thus, it is prudent to evaluate easier and effective methods to screen patients with early changes and subsequently follow-up and halt/treat progression.
At present, the mechanism that has been postulated is increased cerebral blood flow, venous stasis, decreased oxygenation to the brain and capillary leakage.2 As retina has a high oxygen consumption, hypoxia can cause structural and functional changes in the retinal vasculature. The eye is considered the window of the brain, reflecting the intracranial affection in the retina, optic disc and retinal vasculature. Fundus examination gives a direct picture of the state of the retina and its vasculature, and reflects the changes that have occurred in the brain in those with AMS as well as normal individuals at high altitudes. A positive correlation of retinal vascular changes in AMS and normal subjects at high altitudes may therefore support the above-mentioned mechanisms.
Various studies have elucidated retinal changes in healthy subjects at high altitudes, which includes high altitude-associated retinal haemorrhages (HARH), changes in the retinal vasculature calibre, papilloedema and exudates.3 Studies have also highlighted the retinal features in AMS.1–4 However, studies comparing retinal changes at high altitudes in healthy subjects with those in AMS are sparse. Thus, we documented retinal vascular changes in AMS and compared these with healthy subjects after ascent to high altitude. We also explored the effects on retinal vessel regulation of re-entry to high altitude.
METHODS
Study design
We did this cross-sectional observational study at Leh (Ladakh), India. Approval of the Ethical Committee was obtained before the study began. The study protocol conformed to the tenets of the Declaration of Helsinki. Written consent was obtained from all participants after they were given a detailed explanation of the study. All subjects travelled from Chandigarh to Leh by flight, in a time span of 1.5 hours and were working in uniform conditions in Leh. They ascended from an altitude of (709–1053 feet) to an altitude of 11 000 feet. All subjects were men.
Clinical assessment of AMS and fundus changes
The subjects were evaluated for AMS by the Lake Louise protocol.4 To ensure blinding, the same staff were assigned at all times to complete the questionnaires. All subjects were briefed thoroughly regarding the nature of the questionnaires before baseline recordings. Individuals with a self-assessment score of <3 were diagnosed as not having AMS (AMS–) and those with a score of >4 with headache were diagnosed as having AMS (AMS+). Age, time spent at high altitude, absence from high altitude before re-induction (re-induction is re-entry to high altitude and in our study, it was done to join duty after completion of leave), number of times of ascent, history of altitude disorders, migraine and systemic disorders were obtained. SpO2, respiratory rate, pulse rate and blood pressure were recorded. Fundoscopy was done in both the eyes under mydriasis, which was achieved by topical application of tropicamide, 1% w/v eye drops. One drop was instilled in each eye and the fundus was examined after 15 minutes. The findings were recorded on a printed proforma. The fundus examination in all subjects was done by a single observer with a direct ophthalmoscope (Heine beta 200). The retinal findings noted were either retinal vein dilatation with or without tortuosity (Fig. 1a), optic disc oedema, hyperaemic optic disc alone, and optic disc hyperaemia with retinal vein changes (Fig. 1b). All the above procedures were done 24 hours after ascent, i.e. on the morning of day 2 of ascent.
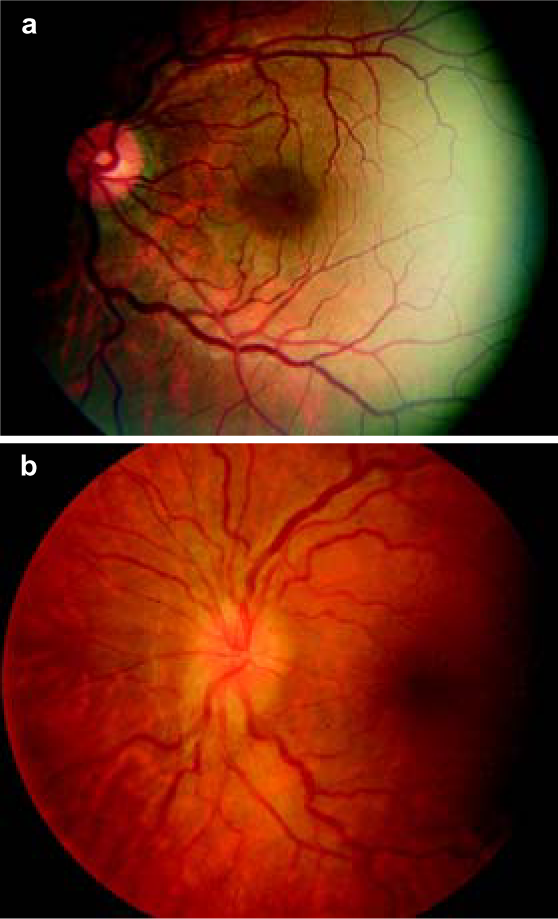
- Colour fundus photograph of a patient with acute mountain sickness showing (a) retinal vein dilatation and tortuosity and (b) optic disc hyperaemia with retinal vein dilatation
Subjects diagnosed as AMS as per the Lake Louise protocol and who had clinical, laboratory or radiological evidence of other concomitant high-altitude diseases such as high-altitude pulmonary or cerebral oedema were excluded. Those with a history of smoking, consumption of alcohol, hypertension, diabetes or other forms of retinopathy, migraine, or any history or clinical evidence of conditions likely to have involvement of the retina, and thereby potentially interfering with changes in the fundus of those with AMS were also excluded from the study.
Statistics
Descriptive statistics were used to describe the distribution of parameters and Fisher exact test was used to determine if there was any association or comparison between two categorical variables. A value of p<0.05 was considered statistically significant.
RESULTS
Of the 97 individuals examined, 43 were AMS– and 54 were AMS+. Those with AMS had a mean age of 29.5 years (range 21–69 years) and those without AMS had a mean age of 32.0 years (range 22–45 years). None of those examined had retinal haemorrhages, cotton wool spots or optic disc oedema.
The average SpO2 was 91% (range 78%–98%) in those with AMS and 91.6% in those without AMS (range 88%–98%). Of the 50 individuals with retinal vein dilatation (both AMS+ and AMS–), 22 (45.8%) had SpO2 >91% and 28 (57.1%) had SpO2 <91% (p<0.1). Among those with AMS and retinal vein dilatation, 17 (50%) had SpO2 >91% and 19 (79.2%) had SpO2 <91% (p<0.01).
Retinal vein dilatation in AMS+ was seen in 36 (66.7%) and in AMS– in 14 (32.6%; p=0.01; Table I).
| Acute mountain sickness | Only retinal vein dilatation (%) |
Normal retinal vein (%) |
Total | No retinal vein tortuosity (%) |
Retinal vein tortuosity (%) |
Total |
|---|---|---|---|---|---|---|
| Present | 36 (66.7) | 18 (33.3) | 54* | 12 (22.2) | 42 (77.8) | 54† |
| Absent | 14 (32.6) | 29 (67.4) | 4 3 | 3 (7.0) | 40 (93.0) | 4 3 |
| Total | 50 (51.6) | 47 (48.4) | 9 7 | 15 (15.5) | 82 (84.5) | 9 7 |
Hyperaemic optic disc in AMS+ was seen in 30 (55.6%) and in AMS– in 14 (32.6%; p=0.03). Hyperaemic optic disc with retinal vein dilatation in AMS+ was seen in 27 (50%) and in AMS– in 9 (20.9%; p=0.006; Table II). The relation of AMS score to retinal vein dilatation is shown in Table III. The average induction number (re-entry into high altitude) was 3.35 for AMS+ and 2.65 for AMS–. The relation of induction number and retinal vein dilatation is shown in Table IV.
| Acute mountain sickness | Hyperaemic optic disc with vein dilatation (%) |
No hyperaemic optic disc with vein dilatation (%) |
Total | Only optic disc hyperaemia (%) |
Normal optic disc (%) |
Total |
|---|---|---|---|---|---|---|
| Present | 27 (50) | 27 (50) | 54* | 30 (55.6) | 24 (44.4) | 54† |
| Absent | 9 (20.9) | 34 (79.1) | 4 3 | 14 (32.6) | 29 (67.4) | 4 3 |
| Total | 36 (37.1) | 61 (62.9) | 9 7 | 44 (45.4) | 53 (54.6) | 9 7 |
| AMS score | ≥5 (%) | <5 (%) | Total |
|---|---|---|---|
| Dilated veins | 25 (69.4) | 11 (30.6) | 36 |
| Normal veins | 09 (50).0 | 09 (50).0 | 18 |
p<0.001
| Acute mountain sickness with retinal vein dilatation |
Induction number | Total | |
|---|---|---|---|
| ≥3 (%) | <3 (%) | ||
| Present | 19 (52.8) | 17 (47.2) | 3 6 |
| Absent | 8 (57.1) | 6 (42.9) | 1 4 |
DISCUSSION
The choroidal circulation, which serves the outer retina, is a high-flow, low oxygen extraction system with low resistance and autonomic vasoactive neurons regulate it. In contrast, the retinal circulation is a relatively low-flow, high oxygen extraction system with no autonomic vasoactive neurons. It is autoregulated by tissue oxygen tension, metabolic factors such as endothelial-derived nitric oxide (NO) released in response to hypoxia, causing vasodilatation. Myogenic factors such as an increase or decrease in transmural pressure causes vasoconstriction or vasodilatation, respectively.5 The blood supply to the retina and the brain behave similarly due to similar vascular regulatory processes. Thus, retinal examination is a reflection of the changes that have occurred in the brain in AMS.
We observed a significant number of AMS+ compared to AMS– with retinal vascular changes in the form of retinal vein dilatation and tortuosity. The increase in the retinal vein’s calibre is presumably due to altered blood flow, i.e. stasis and an increase in arterial blood flow correlating with the altered cerebral blood flow. The tortuosity of the vessels is due to local physical lengthening of the vessel along with dilatation. Local hypoxia and increased retinal blood flow may be responsible for the dilated retinal capillary on the optic disc making it hyperaemic.6 Interestingly, no retinal haemorrhages were seen in any subject. This may be because the hypoxia was not severe enough. Most of the studies that reported HARH7 observed it at an altitude higher than that in our study.
We observed an increased incidence of retinal vein changes in AMS+ compared to AMS–. The difference was statistically significant. This shows, although hypoxia is the main factor in altered retinal vein calibre, the incidence rises in AMS+ due to some other local factors. Thus, we postulate that factors playing a role in the pathogenesis of AMS apart from hypoxia might be free radicals, NO, inflammatory mediators such as vascular endothelial growth factor (VEGF), and cerebral vein dilatation causing retinal vein dilatation.8,9 The severity of AMS correlates well with retinal vein dilatation. A score of >5 was significant for a higher incidence of altered vessel calibre. This further strengthens the postulation that apart from hypoxia, hypoxia-induced blood flow parameters and local factors are brought into play in AMS.
In response to hypobaric hypoxia, there is an initial increase in the retinal blood velocity, which decreases on further ascent. The retinal vessel diameter does not increase with further ascent due to anatomical and mechanical restrictions. However, the choroidal blood flow increases during later stages and at even higher altitudes.
Although there are studies on the relation between retinal vessels and AMS, they had a small sample size.10 Our study evaluated the vessel characteristics qualitatively. The inter-observer bias was reduced as a single person evaluated the subjects. Our study’s strength is the bigger sample size and comparison of the fundus changes in individuals suffering from AMS from those not having AMS. Our study is the first to evaluate the effect of re-entry into high altitude and retinal vein dilatation. A high significance was noted between re-entry for the third time or more and retinal vein dilatation. Thus, after each re-entry, the propensity to develop AMS and altered retinal blood flow and retinal vessel dilatation increases. This probably reflects altered cerebral blood flow in AMS.
To conclude, apart from acute hypobaric hypoxia factors such as free radicals, NO, inflammatory mediators such as VEGF causes retinal venous dilatation, tortuosity, and hyperaemic optic disc in AMS+ with lower SpO2 levels. The incidence of retinal vein dilatation increases with more severe symptoms of AMS and with frequent re-entry into high altitude. The increase in vessel diameter is due to a combination of the central mechanism of increased blood flow and some outflow resistance and peripheral factors in the retina such as reactive oxygen species, NO and VEGF.
Though advanced diagnostics are the gold-standard in the evaluation of such subjects, simpler investigative modalities such as direct ophthalmoscopy can help as a screening tool to evaluate and triage patients for further evacuation, treatment and investigation at a higher centre.
References
- Retinal vessel leakage at high altitude. JAMA. 2013;309:2210-12.
- [CrossRef] [PubMed] [Google Scholar]
- High altitude retinal hemorrhages-an update. High Alt Med Bio. 2012;13:240-4.
- [CrossRef] [PubMed] [Google Scholar]
- The Lake Louise acute mountain sickness scoring system In: Sutton JR, Houston CS, Coates G, eds. Hypoxia and mountain medicine. Burlington, VT: Queen City Printers; 1993.
- [Google Scholar]
- Quantification of optic disc edema during exposure to high altitude shows no correlation to acute mountain sickness. PLoS One. 2011;6:e27022.
- [CrossRef] [PubMed] [Google Scholar]
- Estimated incidence of high altitude retinal hemorrhages. Graefes Arch Clin Exp Ophthalmol. 2018;256:231-2.
- [CrossRef] [PubMed] [Google Scholar]
- The headache of high altitude and microgravity-similarities with clinical syndromes of cerebral venous hypertension. High Alt Med Bio. 2011;12:379-86.
- [CrossRef] [PubMed] [Google Scholar]
- New insights into ocular blood flow at very high altitudes. J Appl Physiol. 2009;106:454-60.
- [CrossRef] [PubMed] [Google Scholar]
- Update on high altitude cerebral edema including recent work on the eye. High Alt Med Bio. 2014;15:122.
- [CrossRef] [PubMed] [Google Scholar]




