Translate this page into:
Favipiravir therapy for Covid-19 infection and tacrolimus toxicity in a kidney transplant patient on chronic eculizumab therapy
Correspondence to MEVLUT TAMER DINCER; tamerdincer@gmail.com
[To cite: Dincer MT, Trabulus S, Seyahi N. Favipiravir therapy for Covid-19 infection and tacrolimus toxicity in a kidney transplant patient on chronic eculizumab therapy. Natl Med J India 2022;35:232–4.]
Abstract
Although the latest data show that complement activation has an essential role in the pathogenesis and severity of Covid-19, the data on the prognosis of patients using complement inhibitors during Covid-19 infection are scarce. There is no specific treatment for Covid-19 yet. The introduction of novel agents such as favipiravir may affect metabolism of immunosuppressive drugs. We report the clinical course of Covid-19 in a kidney transplant patient with atypical haemolytic uraemic syndrome on chronic eculizumab therapy. The patient had mild Covid-19 but had severe tacrolimus toxicity, which may be associated with favipiravir and eculizumab. The mild course of Covid-19 in our patient is encouraging for eculizumab use; on the other hand, unusually high levels of tacrolimus that we observed underlines the importance of frequent drug level monitoring in transplanted patients who are receiving new drugs.
INTRODUCTION
The clinical manifestations of Covid-19 can range from asymptomatic infection, flu-like upper respiratory tract disease to severe viral pneumonia and respiratory failure. Morbidity and mortality due to Covid-19 infection are reported to be higher for kidney transplant recipients compared to the general population, which may be explained by chronic immuno-suppression and coexisting conditions. Timely use of antiviral agents such as favipiravir may be life-saving for such patients, but metabolism of immunosuppressive drugs might be affected.
Studies suggest that the complement system’s activation has a critical role in the pathogenesis of Covid-19, causing endothelial injury and lung damage, and inhibition of the terminal complement pathway may be a beneficial intervention in Covid-19.1,2 Eculizumab is a monoclonal antibody that binds to the C5 complement protein and inhibits the complement pathway. We report Covid-19 in a kidney transplant recipient who was on chronic eculizumab therapy due to atypical haemolytic uraemic syndrome (aHUS) and who had tacrolimus toxicity after the introduction of favipiravir.
THE CASE
A 59-year-old woman, who had received a living donor kidney transplant, was admitted to our hospital with fever, malaise and cough of 3 days duration. She had no history of smoking, cardiovascular or pulmonary disease. The cause of her kidney failure was aHUS. The diagnosis was made at another hospital in 2014, and she had been on eculizumab 900 mg every 2 weeks since then. However, no complement molecule-associated mutation was identified.
She had a paired kidney transplantation 14 months ago. Her husband was the prospective donor; however, she had high levels of pre-existing donor-specific antibodies towards him. Thus, a paired kidney transplantation was preferred with another pair who were siblings. The patient received induction immunosuppression with anti-thymocyte globulin and methylprednisolone. Her maintenance immunosuppressive therapy was tacrolimus (4.5 mg/day), mycophenolate mofetil (1000 mg/day) and prednisolone (5 mg/day). Her renal functions were stable, with a serum creatinine of 1.3 mg/dl.
On admission to our hospital, she was haemodynamically stable (blood pressure 120/70 mmHg, pulse rate 82 per minute). Oxygen saturation was 98% on room air. Other aspects of her physical examination were unremarkable. Laboratory investigations showed that her serum urea was 37 mg/dl (range 17–49 mg/dl), creatinine 1.43 mg/dl, sodium 135 mmol/L, potassium 5.02 mmol/L, calcium 8.6 mg/dl, albumin 3.59 g/dl, white blood cell count 3400/cmm, total lymphocyte count 900/ cmm, haemoglobin 11.3 g/dl, platelet count 139 600/cmm, C-reactive protein 49.7 mg/L (normal <5 mg/L), D-dimer 1.2 mg/L (normal <0.5 mg/L), alanine aminotransferase 72.8 i.u./L (normal <33 i.u./L). Complete urine analysis was unremarkable. Creatine phosphokinase, C3, C4, haptoglobin and procalcitonin levels were within normal limits (Table I). Reverse transcription polymerase chain reaction for SARS-CoV-2 from combined oral and nasal swab was positive. Chest computed tomography revealed bilateral multifocal ground-glass opacities and lung involvement was moderate according to the Radiological Society of North America (RSNA) consensus statement for Covid-19 imaging (Fig. 1).3 She had mild Covid-19 pneumonia without consistent hypoxaemia. Mycophenolate was discontinued and the dose of prednisolone was increased to 10 mg/day. Favipiravir was prescribed as the antiviral therapy on the day of admission with a loading dose of 1600 mg b.d. on day 1, followed by 600 mg b.d. for 6 days. In addition to favipiravir, low molecular weight heparin was administered for thromboprophylaxis. The patient did not receive any other medication, antibiotics or supplements.
| Variable | Day 0 | Day 3 | Day 7 | Day 11 |
|---|---|---|---|---|
| Serum creatinine (mg/dl) | 1.43 | 1.63 | 1.96 | 1.76 |
| Sodium (mEq/L) | 135 | 135 | 130 | 134 |
| Potassium (mEq/L) | 5.02 | 4.91 | 5.4 | 5.12 |
| Albumin (g/dl) | 3.59 | 3.51 | 3.25 | 3.27 |
| White blood cell count/cmm | 3400 | 6400 | 5600 | 6600 |
| Lymphocytes/cmm | 900 | 600 | 600 | 700 |
| Haemoglobin (g/dl) | 11.3 | 11.1 | 10.8 | 11.2 |
| Platelets/cmm | 139 600 | 162 000 | 154 000 | 149 000 |
| Lactate dehydrogenase (i.u./L) | 339 | 330 | 320 | 354 |
| Haptoglobin (mg/dl) | 354 | – | 413 | – |
| C3 (mg/dl) | 1.26 | – | 1.38 | – |
| C4 (mg/dl) | 0.38 | – | 0.4 | – |
| C-reactive protein (mg/L) | 49.7 | 48.2 | 61.8 | 11.6 |
| Procalcitonin (ng/ml) | 0.14 | 0.12 | 0.16 | 0.07 |
| D-dimer (mg/L) | 1.20 | 1.09 | 0.59 | 0.54 |
| Ferritin (ng/ml) | 814.4 | 808.2 | 723.6 | 625.3 |

- Unenhanced chest computed tomography on admission showing bilateral multifocal ground-glass opacities
On day 2 of her admission, the scheduled eculizumab infusion (1200 mg) was administered. Her oxygen saturation ranged between 94% and 98% during the course, and the patient needed intermittent nasal oxygen. By the end of the first week of admission, the patient’s clinical picture improved, but the serum creatinine levels showed a progressive upward trend reaching 1.96 mg/dl (Fig. 2). Concurrently, the tacrolimus trough level reached 30 ng/ml, and tacrolimus needed to be discontinued for 4 days until the trough levels decreased to the therapeutic range. The patient was euvolaemic with adequate diuresis. She was not on any nephrotoxic drugs. She did not have any signs of sepsis and she was haemodynamically stable throughout her hospital stay. The most likely reason for acute kidney injury in our patient was acute tacrolimus nephrotoxicity. In addition, her serum creatinine levels started to decrease after stopping of tacrolimus and returned to baseline 4 days after discharge (Fig. 2).
DISCUSSION
This patient is interesting because of two distinctive features. First, there are only a few reports of Covid-19 infection in renal transplant patients who are on eculizumab, and the course of Covid-19 under eculizumab treatment is not well defined. Second, the patient had severe tacrolimus toxicity during treatment, an event that has not been reported earlier with favipiravir or eculizumab use alone. The combined use of these two drugs might be associated with the development of tacrolimus toxicity, which may be a relevant point for transplant clinicians.
In the recent literature, there are a few reports of individuals already receiving maintenance C5 inhibitors (eculizumab or ravulizumab) during Covid-19 infection.4–8 Most of those patients were using C5 inhibitors for the treatment of paroxysmal nocturnal haemoglobinuria,4,6–8 and only two patients with kidney transplants5,7 were on eculizumab therapy (Table II). Trimarchi et al. reported a kidney transplant recipient with aHUS on maintenance eculizumab therapy. Although the patient had received eculizumab in the early stage of the disease, he developed severe Covid-19, was transferred to the intensive care unit and finally recovered following dexamethasone and convalescent plasma therapy.5 Araten et al. reported three patients, and one of them was a kidney transplant patient on chronic eculizumab therapy for post-transplant thrombotic microangiopathy. Total haemolytic complement (CH50) levels measured on admission suggested eculizumab to be at therapeutic levels, and the patient had mild Covid-19.7
Emerging data show that complement activation plays an essential role in the pathogenesis and severity of Covid-19.9 The complement system activation leads to the formation of chemotactic and anaphylatoxic C5a, which has a crucial role in activating phagocytic cells and the generation of cytokine storm.10 Magro et al. examined post-mortem findings in 5 Covid-19 patients and found a deposition of C5b-9, C4d and mannose-binding lectin-associated serine protease 2 in the pulmonary and cutaneous capillary circulation, related to profound and generalized activation of the complement pathways.11 The potential use of therapeutic complement inhibitors is currently being studied for Covid-19 (NCT04346797, NCT04355494, NCT04288713, NCT04390464, NCT04570397 and NCT04369469). Evidence from recent papers suggests that patients on maintenance anti-complement therapy might be protected from the damage caused by the virus.9 However, the complement system is part of the innate immunity and efficiently recognizes and eliminates viral pathogens, and there is a concern about their use in the early stage of Covid-19.12
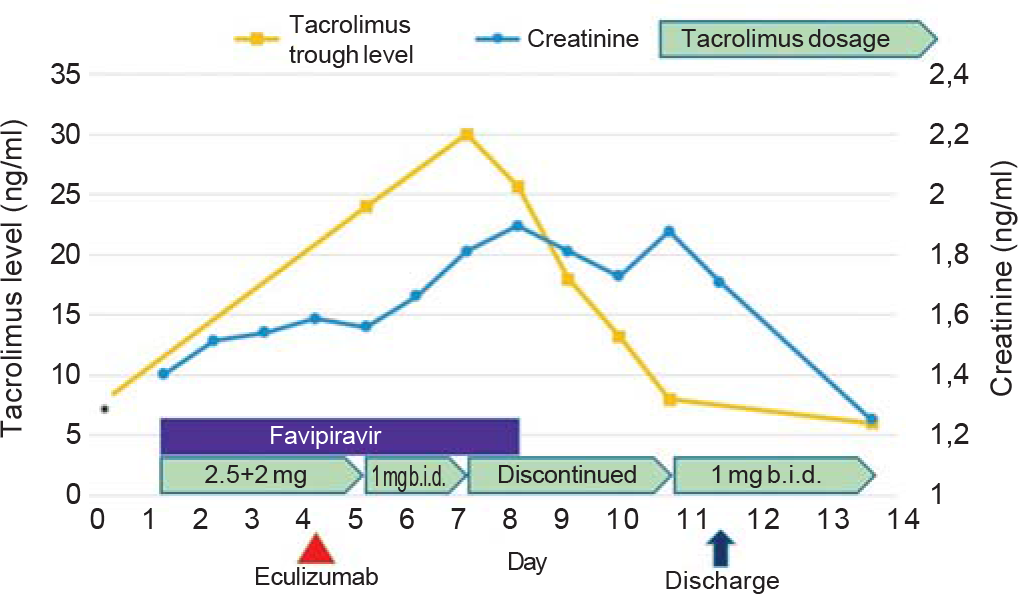
- Serum tacrolimus and creatinine levels, and treatment of the patient. *The last measured tacrolimus level (3 months ago) before admission
| Reference number | Age (gender) | Kidney transplant | Type of C5 inhibitor | Indication for C5 inhibitor | Initiated treatment | Covid-19 outcome |
|---|---|---|---|---|---|---|
| [4] | 35 (woman) | No | Ravulizumab | PNH | – | Recovered |
| [4] | 37 (woman) | No | Eculizumab | PNH | – | Recovered |
| [5] | 24 (man) | Yes | Eculizumab | aHUS | Antibiotics, dexamethasone, convalescent | Recovered |
| plasma infusion | ||||||
| [6] | 61 (woman) | No | Ravulizumab | PNH | Antibiotics | Recovered |
| [6] | 47 (man) | No | Eculizumab | PNH | Antibiotics | Recovered |
| [6] | 43 (man) | No | Eculizumab | PNH | Antibiotics | Died |
| [6] | 77 (woman) | No | Eculizumab | PNH | Antibiotics | Recovered |
| [7] | 39 (woman) | No | Ravulizumab | PNH | Antibiotics | Recovered |
| [7] | 54 (woman) | Yes | Eculizumab | Post-transplant TMA | – | Recovered |
| [7] | 60 (woman) | No | Eculizumab | PNH | – | Recovered |
| [8] | 45 (man) | No | Eculizumab | PNH | Antibiotics, hydroxychloroquine, | Recovered |
| lopinavir/ritonavir, tocilizumab, enoxaparin | ||||||
| Our patient | 59 (woman) | Yes | Eculizumab | aHUS | Favipiravir, enoxaparin | Recovered |
aHUS Favipiravir, enoxaparin Recovered aHUS atypical haemolytic uraemic syndrome PNH paroxysmal nocturnal haemoglobinuria TMA thrombotic microangiopathy
At present, there is no specific treatment for kidney transplant recipients with Covid-19. Virus replication is evident in the early stage of infection; therefore, reducing immunosuppression can be considered.13 Different countries have adopted various treatment modalities according to the available evidence and resources. Favipiravir use was suggested in the guideline of Covid-19 management prepared by the Turkish coronavirus scientific committee. This guideline was published by the Ministry of Health of the Republic of Turkey and was widely adopted by physicians. Co-administration of tacrolimus and favipiravir has not been studied yet, and to our knowledge, no drug interaction with tacrolimus and favipiravir has been reported. Tacrolimus is mainly metabolized in the liver by CYP3A4 and CYP3A5 enzymes.14 On the other hand, favipiravir is metabolized in the liver by aldehyde oxidase.15 However, there may be common pathways in the metabolism of tacrolimus and favipiravir that are not fully explored yet. As an example, favipiravir is a weak inhibitor of the CYP2C8 system and it has been previously shown that genetic polymorphisms in CYP2C8 may exacerbate tacrolimus toxicity.14 We should emphasize that, as far as we know, eculizumab use is not associated with tacrolimus toxicity.
In conclusion, our report adds to the clinical management of Covid-19 patients. The mild Covid-19 in our patient is encouraging for the use of eculizumab; on the other hand, unusually high levels of tacrolimus that we observed underline the importance of frequent drug level monitoring in transplant patients who are receiving new drugs.
Conflicts of interest
None declared
References
- Complement as a target in COVID-19? Nat Rev Immunol. 2020;20:343-4.
- [CrossRef] [PubMed] [Google Scholar]
- Eculizumab as an emergency treatment for adult patients with severe COVID-19 in the intensive care unit: A proof-of-concept study. E Clin Med. 2020;28:100590.
- [CrossRef] [PubMed] [Google Scholar]
- Radiological Society of North America expert consensus statement on reporting chest CT findings related to COVID-19. Endorsed by the Society of Thoracic Radiology the American College of Radiology, and RSNA-Secondary Publication. J Thorac Imaging. 2020;35:219-27.
- [CrossRef] [PubMed] [Google Scholar]
- Terminal complement inhibition dampens the inflammation during COVID-19. Br J Haematol. 2020;190:e141-3.
- [CrossRef] [Google Scholar]
- Eculizumab, SARSCoV-2 and atypical hemolytic uremic syndrome. Clin Kidney J. 2020;13:739-41.
- [CrossRef] [PubMed] [Google Scholar]
- COVID-19 infection in patients on anti-complement therapy: The Leeds National Paroxysmal Nocturnal Haemoglobinuria service experience. Br J Haematol. 2020;191:e1-e4.
- [CrossRef] [PubMed] [Google Scholar]
- Mild clinical course of COVID-19 in 3 patients receiving therapeutic monoclonal antibodies targeting C5 complement for hematologic disorders. Am J Case Rep. 2020;21:e927418.
- [CrossRef] [PubMed] [Google Scholar]
- Severe COVID-19 infection in a patient with paroxysmal nocturnal hemoglobinuria on eculizumab therapy. Leuk Lymphoma. 2021;62:1-4.
- [CrossRef] [PubMed] [Google Scholar]
- The case of complement activation in COVID-19 multiorgan impact. Kidney Int. 2020;98:314-22.
- [CrossRef] [PubMed] [Google Scholar]
- The role of C5a in acute lung injury induced by highly pathogenic viral infections. Emerg Microbes Infect. 2015;4:e28.
- [CrossRef] [PubMed] [Google Scholar]
- Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. Transl Res. 2020;220:1-13.
- [CrossRef] [PubMed] [Google Scholar]
- The complement system in COVID-19: Friend and foe? JCI Insight. 2020;5:e140711.
- [CrossRef] [PubMed] [Google Scholar]
- COVID-19 in renal transplant recipients: Case series and a brief review of current evidence. Nephron. 2021;145:192-8.
- [CrossRef] [PubMed] [Google Scholar]
- Pharmacogenetic aspects of the use of tacrolimus in renal transplantation: Recent developments and ethnic considerations. Expert Opin Drug Metab Toxicol. 2016;12:555-65.
- [CrossRef] [PubMed] [Google Scholar]
- Pharmaceuticals and Medical Devices Agency: Avigan (favipiravir) Review Report. 2014. Available at www.pmda.go.jp/files/000210319.pdf (accessed on 15 Jul 2021)
- [Google Scholar]




