Translate this page into:
Scrub typhus presenting with massive splenomegaly and lobar pneumonia
Correspondence to JONNALAGADDA VIHARI; viharijtk5@gmail.com
[To cite: Vihari J, Aditya A, Krishna AV, Roja T, Elika M, Pooja U. Scrub typhus presenting with massive splenomegaly and lobar pneumonia. Natl Med J India 2024:37:200–2. DOI: 10.25259/NMJI_1001_2021]
Abstract
Scrub typhus is still an underdiagnosed disease despite an increase in incidence as the clinical presentation is often different, leading to a low index of suspicion among doctors. Scrub typhus, an acute febrile disease, is a cause of prolonged fever and pyrexia of unknown origin. It can have varied clinical presentations ranging from mild asymptomatic disease to fatal multi-organ dysfunction. Splenomegaly in scrub typhus has been rarely reported. We report a 30-year-old man presenting with fever, hepatomegaly, massive splenomegaly, lymphadenopathy and lobar pneumonia. Tests for malarial parasite and enteric fever were negative. Bone marrow aspiration showed normal haematopoiesis. IgM scrub was positive. Upon serological confirmation, doxycycline therapy was started followed by a rapid and complete resolution of pneumonia (both clinically and radiologically), splenomegaly and lymphadenopathy. This highlights the importance of recognizing rare clinical manifestations of this common tropical disease. An early diagnosis is required as a delay may lead to complications and a poor outcome.
INTRODUCTION
Scrub typhus, also known as tsutsugamushi disease, is a bacterial infection transmitted by larval trombiculid mites from rodents often during winter months. The causative organism is Orientia tsutsugamushi, an obligatory intracellular bacterium that leads to the formation of eschar at the inoculation site followed by fever, headache, myalgia, generalized lymphadenopathy, cough, gastrointestinal symptoms, transient hearing loss and rash.1 Further progression of the disease may manifest as acute respiratory distress syndrome, meningoencephalitis, gastrointestinal bleeding, acute renal failure and coagulopathy.2 Scrub typhus is also a possible cause of pyrexia of unknown origin (PUO).3–5 Hepatosplenomegaly is not an uncommon occurrence in scrub typhus-positive patients. Massive splenomegaly in isolated scrub typhus infection has been rarely reported in the literature. Scrub typhus is still underdiagnosed in spite of increasing incidence due to the varied clinical presentations of the disease and a high index of suspicion is required for the diagnosis. We report a patient with massive splenomegaly and lobar pneumonia due to scrub typhus, which is a rare presentation.
THE CASE
A 30-year-old man, resident of Berhampur, Ganjam district of Odisha state, working as an agricultural daily labourer, was admitted with a history of fever with chills, headache, cough with scanty expectoration, and myalgia for 5 days. The fever was continuous, high grade and associated with severe headache in the retro-orbital location with no associated visual disturbances. The patient had breathlessness with Modified Medical Research Council (MMRC) dyspnoea scale Grade 1. He lived in a semi-urban area with no history of any insect bite.
He did not have any past medical history and he was not on any medications previously. There was no history of similar complaints in any of his family members. There was no personal history of smoking and alcohol consumption. On examination, he was febrile (temperature 102 °F), dehydrated and looking toxic, tachypnoeic (28 breaths/minute), and had a pulse rate of 104/minute and blood pressure of 98/58 mmHg with no skin rashes or eschar. There was no icterus, cyanosis, pallor, clubbing or pedal oedema. His jugular venous pressure was not raised. The patient had multiple, firm to rubbery, non-tender, discrete lymph nodes in the axillary region (largest in the posterior axillary area measuring 1.5×2 cm). He also had palpable firm, non-tender, discrete lymph nodes in the inguinal region around 1.5×2 cm in diameter. Systemic examination revealed tubular bronchial breathing in the left supraclavicular, infraclavicular, axillary and suprascapular areas along with fine crepitations and diminished breath sounds in basal regions of the left side with SpO2 of 88%–94% at room air with normal cardiovascular status. Abdominal examination revealed prominent splenomegaly, palpable up to 11 cm below the costal margin and mild hepatomegaly (liver span 15 cm); neurological examination was normal. The patient was diagnosed clinically to have left upper lobe consolidation due to community-acquired pneumonia and started on injection ceftriaxone 1 g, intravenous twice daily, suspecting bacterial aetiology along with oxygen after sending relevant investiga-tions. Arterial blood gas analysis showed PaO2 61 mmHg, PaCO2 29 mmHg, pH 7.55, HCO3 22 mEq/L, suggesting hypo-xaemia with respiratory alkalosis with a CURB-65 score of 1.
Complete blood counts revealed mild normocytic normochromic anaemia (haemoglobin 11 g/dl), thrombocytopenia (96 000 cells/cmm) and normal leucocyte count 8400/cmm. The C-reactive protein was raised (86 mg/dl), normal electrolytes, renal function tests, liver function tests, sterile blood cultures, negative for malarial parasite in immunochromatographic assay and IgM dengue. Chest X-ray on admission revealed left upper and mid-zone heterogenous opacities with air bronchogram (Fig. 1) and mild blunting of the left costophrenic angle. Ultrasound abdomen showed massive splenomegaly (Fig. 2). Contrary to our expectations, investigations revealed a normal white blood cell count with sputum negative for Gram-stain and no growth in culture, which is unusual in a case of bacterial pneumonia. Because of upper lobe involvement, suspecting pulmonary tuberculosis, sputum was collected and sent for acid-fast bacilli, CBNAAT (cartridge-based nucleic acid amplification test)—both were negative. Despite 2 days of antibiotics the patient’s fever persisted, tachypnoea worsened and he became hypotensive (78/50 mmHg). The patient developed puffiness of the face and pedal oedema on day 5 of admission. His oxygen saturation dropped to 88% on room air, while urine output was adequate. Urinalysis revealed trace proteinuria (9.8 mg/dl), pyuria (5–8 white blood cells per high power field) but no haematuria. Urine culture was sterile. ECG showed sinus tachycardia. There was no evidence of vegetations or valvular regurgitations on 2-dimensional echocardiogram. CT scan of the abdomen and thorax was done, which showed mild hepatomegaly, gross splenomegaly, left upper and lingular lobe consolidation with air bronchogram, mild left subpulmonic pleural effusion with collapse in the basal region of the left lung (Fig. 3). Facial puffiness and pedal oedema resolved on diuretic treatment by day 12.
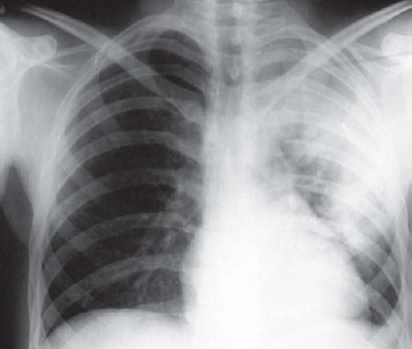
- Chest X-ray on admission showing the left upper region and mid-zone heterogeneous opacities with air bronchogram
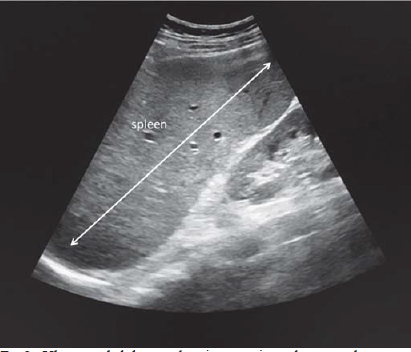
- Ultrasound abdomen showing massive splenomegaly
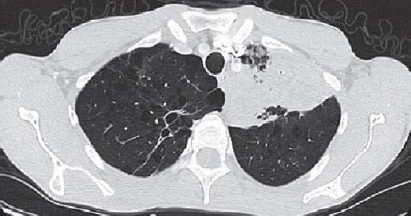
- Computed tomography of the chest showing left upper lobe consolidation
A bone marrow aspiration done on day 7 to exclude the possibility of lymphoma showed normal haemopoeisis without any immature cells. In view of the non-resolving fever, thrombocytopenia, hepatosplenomegaly and lung consolidation, IgM antibody for scrub typhus was done, which was positive. Lymph node biopsy was not done suspecting the enlargements to be reactive changes. Oral doxycycline 100 mg b.d. was started on day 12 and in 24 hours the fever spikes reduced and the respiratory distress improved, pyrexia resolved on day 2 of treatment. By day 18, the spleen regressed completely and lymph nodes in the axillary and inguinal regions were no longer palpable. Chest X-ray before discharge showed complete resolution of opacities, which is rare, as radiological findings due to pneumonia lag behind clinical improvement by few weeks. The patient was discharged on day 20 post-admission. His oral doxycycline was continued for 5 more days. He was asymptomatic on a follow-up visit after a week.
DISCUSSION
Scrub typhus is an acute febrile illness caused by the obligate intracellular Gram-negative bacterium Orientia tsutsugamushi transmitted to rodents (primary host) and humans (accidental host) by the bite of the larval form of trombiculid mite. Globally, over one billion people are at risk and an estimated one million cases of scrub typhus occur annually.6 It is reported to be increasing and accounts for up to 50% of undifferentiated fever presenting to hospital in some regions of India.7 The mortality ranges from 30% to 45% if not diagnosed promptly and treated in endemic countries.8 Scrub typhus can have diverse clinical manifestations ranging from fever, headache, myalgia, generalized or regional lymphadenopathy to meningoencephalitis, acute respiratory distress syndrome and multiple organ dysfunction syndrome with hepatic and renal failure. The classical ‘eschar’ as described in scrub typhus is found only in a few patients.
The Indian subcontinent represents one of the largest tropical and subtropical regions with high prevalence of many of these infections.9 Hepatomegaly and splenomegaly may be found in patients of scrub typhus as reported in previous studies.10–13 Diseases associated with massive splenomegaly include chronic myeloid leukaemia, lymphomas, hairy cell leukaemia, polycythemia vera, sarcoidosis and malaria. Massive splenomegaly in scrub typhus is rarely reported in the literature. Airspace consolidation is relatively uncommon and generally appears in the lower zone of both lungs. Our patient had a massive airspace consolidation involving the left upper and lingular lobes, which is an unusual pulmonary manifestation of scrub typhus and has been reported only once in the literature.14
Our patient had fever, moderate hepatomegaly, massive splenomegaly, and axillary and inguinal lymphadenopathy with left upper lobe consolidation on presentation. The closest differential that we considered was bacterial pneumonia and lymphoma. Normal blood counts, sputum reports, peripheral blood examination, gradual resolution of lymphadenopathy and a normal bone marrow examination ruled these out. A 2D echocardiography done to rule out infective endocarditis, showed an absence of valvular regurgitation or vegetations. Fever in our patient was unresponsive to conventional broad spectrum antibacterials. In view of prolonged non-responsive fever and high endemicity, we ordered IgM antibody for scrub typhus by ELISA, which showed a positive result. Our patient had transient anasarca that could be due to capillary leak induced by the infection. A course of oral doxycycline resolved the fever, hepatosplenomegaly and lymphadenopathy in our patient dramatically, which further confirmed our diagnosis.
Conclusion
Lobar pneumonia, which is commonly due to community-acquired pneumonia of bacterial aetiology, can also be a presenting feature of scrub typhus and physicians should be aware of such an atypical presentation. We should always keep scrub typhus in our differential diagnoses in a febrile patient with splenomegaly who is not responding to routine antibacterials. Conventional antibiotics for the treatment of pneumonia might not be useful in them and delay in the starting of appropriate antibiotics often leads to rapid deterioration in the clinical condition. In endemic regions, one should look for eschar on physical examination carefully as it is a specific clinical sign of scrub typhus that can guide the clinician and in its absence, serological testing should be done in atypical clinical presentations as scrub typhus can present with multiple clinical manifestations.
Conflicts of interest
None declared
References
- Scrub typhus strikes back: Are we ready? Med J Armed Forces India. 2019;75:8-17.
- [CrossRef] [Google Scholar]
- Scrub typhus: Clinical, pathologic, and imaging findings. Radiographics. 2007;27:161-72.
- [CrossRef] [Google Scholar]
- Scrub typhus: Epidemiology, clinical presentation, diagnostic approach, and outcomes. J Indian Acad Clin Med. 2019;20:15-21.
- [Google Scholar]
- Epidemiological and clinical features of scrub typhus in Odisha, Eastern India. Med J Dr DY Patil Vidyapeeth. 2019;12:419-23.
- [CrossRef] [Google Scholar]
- Scrub typhus-an emerging entity: A study from a tertiary care hospital in North India. Indian J Public Health. 2014;58:281-3.
- [CrossRef] [Google Scholar]
- Scrub typhus: The geographic distribution of phenotypic and genotypic variants of Orientia tsutsugamushi. Clin Infect Dis. 2009;48(Suppl 3):S203-S230.
- [CrossRef] [Google Scholar]
- Scrub typhus: Prevalence and diagnostic issues in rural southern India. Clin Infect Dis. 2004;39:1395-6.
- [CrossRef] [Google Scholar]
- Effects of environmental change on emerging parasitic diseases. Int J Parasitol. 2000;30:1395-405.
- [CrossRef] [Google Scholar]
- Scrub typhus in children at a tertiary hospital in southern India: Clinical profile and complications. J Infect Public Health. 2012;5:82-8.
- [CrossRef] [Google Scholar]
- Clinical profile of scrub typhus in children. Indian J Pediatr. 2012;79:1459-62.
- [CrossRef] [Google Scholar]
- Paediatric scrub typhus in Thailand: A study of 73 confirmed cases. Trans R Soc Trop Med Hyg. 2004;98:354-9.
- [CrossRef] [Google Scholar]
- A study on clinico-laboratory parameters of children with scrub typhus in Garhwal region of Himalayan belt. Int J Pediatr Res. 2019;6:91-6.
- [CrossRef] [Google Scholar]
- Massive consolidation: A rare manifestation of paediatric scrub typhus. BMJ Case Reports. 2014;2014 bcr2013200687
- [CrossRef] [Google Scholar]




